Trastuzumab Deruxtecan in Patients With HER2-Mutant Metastatic Non-Small-Cell Lung Cancer: Primary Results From the Randomized, Phase II DESTINY-Lung02 Trial
- PMID: 37694347
- PMCID: PMC10617843
- DOI: 10.1200/JCO.23.01361
Trastuzumab Deruxtecan in Patients With HER2-Mutant Metastatic Non-Small-Cell Lung Cancer: Primary Results From the Randomized, Phase II DESTINY-Lung02 Trial
Erratum in
-
Erratum: Trastuzumab Deruxtecan in Patients With HER2-Mutant Metastatic Non-Small-Cell Lung Cancer: Primary Results From the Randomized, Phase II DESTINY-Lung02 Trial.J Clin Oncol. 2024 Feb 1;42(4):485. doi: 10.1200/JCO.23.02574. Epub 2023 Dec 19. J Clin Oncol. 2024. PMID: 38113428 No abstract available.
-
Erratum: Trastuzumab Deruxtecan in Patients With HER2-Mutant Metastatic Non-Small-Cell Lung Cancer: Primary Results From the Randomized, Phase II DESTINY-Lung02 Trial.J Clin Oncol. 2024 Oct 20;42(30):3635. doi: 10.1200/JCO-24-01883. Epub 2024 Sep 13. J Clin Oncol. 2024. PMID: 39270141 No abstract available.
Abstract
Purpose: Trastuzumab deruxtecan (T-DXd) 5.4 and 6.4 mg/kg showed robust antitumor activity in multiple cancer indications; however, T-DXd 5.4 mg/kg has not been evaluated in patients with previously treated human epidermal growth factor receptor 2-mutant (HER2m; defined as single-nucleotide variants and exon 20 insertions) metastatic non-small-cell lung cancer (mNSCLC).
Methods: DESTINY-Lung02, a blinded, multicenter, phase II study, investigated T-DXd 5.4 mg/kg once every 3 weeks for the first time in previously treated (platinum-containing therapy) patients with HER2m mNSCLC and further assessed T-DXd 6.4 mg/kg once every 3 weeks in this population. The primary end point was confirmed objective response rate (ORR) per RECIST v1.1 by blinded independent central review.
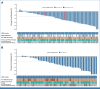
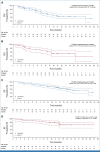
Results: One hundred fifty-two patients were randomly assigned 2:1 to T-DXd 5.4 or 6.4 mg/kg once every 3 weeks. As of December 23, 2022, the median duration of follow-up was 11.5 months (range, 1.1-20.6) with 5.4 mg/kg and 11.8 months (range, 0.6-21.0) with 6.4 mg/kg. Confirmed ORR was 49.0% (95% CI, 39.0 to 59.1) and 56.0% (95% CI, 41.3 to 70.0) and median duration of response was 16.8 months (95% CI, 6.4 to not estimable [NE]) and NE (95% CI, 8.3 to NE) with 5.4 and 6.4 mg/kg, respectively. Median treatment duration was 7.7 months (range, 0.7-20.8) with 5.4 mg/kg and 8.3 months (range, 0.7-20.3) with 6.4 mg/kg. Grade ≥ 3 drug-related treatment-emergent adverse events occurred in 39 of 101 (38.6%) and 29 of 50 (58.0%) patients with 5.4 and 6.4 mg/kg, respectively. 13 of 101 (12.9%) and 14 of 50 (28.0%) patients had adjudicated drug-related interstitial lung disease (2.0% grade ≥ 3 in each arm) with 5.4 and 6.4 mg/kg, respectively.
Conclusion: T-DXd demonstrated clinically meaningful responses at both doses. Safety profile was acceptable and generally manageable, favoring T-DXd 5.4 mg/kg.
Trial registration: ClinicalTrials.gov NCT04644237.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures
Comment in
-
Optimizing Dosing of Trastuzumab Deruxtecan in HER2-Mutant Non-Small-Cell Lung Cancer: A Reminder That More Is Not Always Better.J Clin Oncol. 2023 Nov 1;41(31):4849-4851. doi: 10.1200/JCO.23.01768. Epub 2023 Sep 11. J Clin Oncol. 2023. PMID: 37694345 No abstract available.
References
-
- Mazières J, Barlesi F, Filleron T, et al. : Lung cancer patients with HER2 mutations treated with chemotherapy and HER2-targeted drugs: Results from the European EUHER2 cohort. Ann Oncol 27:281-286, 2016 - PubMed
-
- Peters S, Curioni-Fontecedro A, Nechushtan H, et al. : Activity of afatinib in heavily pretreated patients with ERBB2 mutation-positive advanced NSCLC: Findings from a global named patient use program. J Thorac Oncol 13:1897-1905, 2018 - PubMed
-
- Zhou C, Li X, Wang Q, et al. : Pyrotinib in HER2-mutant advanced lung adenocarcinoma after platinum-based chemotherapy: A multicenter, open-label, single-arm, phase II study. J Clin Oncol 38:2753-2761, 2020 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

