Age-Dependent Increase in Tau Phosphorylation at Serine 396 in Huntington's Disease Prefrontal Cortex
- PMID: 37694372
- PMCID: PMC11823699
- DOI: 10.3233/JHD-230588
Age-Dependent Increase in Tau Phosphorylation at Serine 396 in Huntington's Disease Prefrontal Cortex
Abstract
Background: To date, it is still controversial whether tau phosphorylation plays a role in Huntington's disease (HD), as previous studies demonstrated either no alterations or increases in phosphorylated tau (pTau) in HD postmortem brain and mouse models.
Objective: The goal of this study was to determine whether total tau and pTau levels are altered in HD.
Methods: Immunohistochemistry, cellular fractionations, and western blots were used to measure total tau and pTau levels in a large cohort of HD and control postmortem prefrontal cortex (PFC). Furthermore, western blots were performed to assess tau, and pTau levels in HD and control isogenic embryonic stem cell (ESC)-derived cortical neurons and neuronal stem cells (NSCs). Similarly, western blots were used to assess tau and pTau levels in HttQ111 and transgenic R6/2 mice. Lastly, total tau levels were assessed in HD and healthy control plasma using Quanterix Simoa assay.
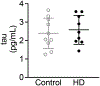
Results: Our results revealed that, while there was no difference in total tau or pTau levels in HD PFC compared to controls, the levels of tau phosphorylated at S396 were increased in PFC samples from HD patients 60 years or older at time of death. Additionally, tau and pTau levels were not changed in HD ESC-derived cortical neurons and NSCs. Similarly, total tau or pTau levels were not altered in HttQ111 and transgenic R6/2 mice compared to wild-type littermates. Lastly, tau levels were not changed in plasma from a small cohort of HD patients compared to controls.
Conclusions: Together these findings demonstrate that pTau-S396 levels increase significantly with age in HD PFC.
Keywords: ESC-derived neurons; Huntington’s disease; fractionations; mouse models; neuronal stem cells; phosphorylated tau; plasma; postmortem brain; total tau.
Conflict of interest statement
P.K. is named as co-inventor on a U.S. patent application related to neurological biomarker assays that is jointly held by Massachusetts General Hospital and Meso Scale Diagnostics. R.M.P. has received sponsored research funding from Pfizer Inc. None of this had any influence over this manuscript.
Figures
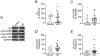
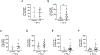


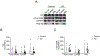


Update of
-
Age-dependent increase in tau phosphorylation at serine 396 in Huntington's disease pre-frontal cortex.medRxiv [Preprint]. 2023 Jun 5:2023.06.03.23290851. doi: 10.1101/2023.06.03.23290851. medRxiv. 2023. Update in: J Huntingtons Dis. 2023;12(3):267-281. doi: 10.3233/JHD-230588. PMID: 37333415 Free PMC article. Updated. Preprint.
References
-
- A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. The Huntington’s Disease Collaborative Research Group. Cell. 1993;72(6):971–83. - PubMed
-
- Ross CA, Aylward EH, Wild EJ, Langbehn DR, Long JD, Warner JH, et al. Huntington disease: natural history, biomarkers and prospects for therapeutics. Nat Rev Neurol. 2014;10(4):204–16. - PubMed
-
- St-Amour I, Turgeon A, Goupil C, Planel E, Hebert SS. Co-occurrence of mixed proteinopathies in late-stage Huntington’s disease. Acta Neuropathol. 2018;135(2):249–65. - PubMed
-
- Fernandez-Nogales M, Cabrera JR, Santos-Galindo M, Hoozemans JJ, Ferrer I, Rozemuller AJ, et al. Huntington’s disease is a four-repeat tauopathy with tau nuclear rods. Nat Med. 2014;20(8):881–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

