Advancing precision oncology through systematic germline and tumor genetic analysis: The oncogenetic point of view on findings from a prospective multicenter clinical trial of 666 patients
- PMID: 37694493
- PMCID: PMC10557826
- DOI: 10.1002/cam4.6498
Advancing precision oncology through systematic germline and tumor genetic analysis: The oncogenetic point of view on findings from a prospective multicenter clinical trial of 666 patients
Abstract
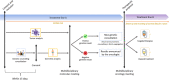
Introduction: With the emergence of targeted therapies, there is a need to accurately identify more tumor biomarkers. The EXOMA trial was designed to offer tumor and germline exome sequencing (ES) to patients with solid malignant tumors and facing therapeutic failure. As hereditary cancer predispositions could be identified, with genetic counseling and health management implications, a genetic consultation was systematically established. This design needs to be discussed as genetic human resources are limited and indication of theranostic tests will increase.
Methods: Genetic counseling was conducted within 15 days following inclusion in the study for patients recruited between December 2015 and July 2019. In silico analyses from theranostic ES were limited to 317 genes involved in oncogenesis, from both tumor and blood DNA.
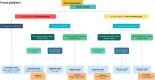
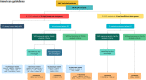
Results: Six hundred and sixty six patients had a genetic consultation before ES. In 65/666 patients, 66 germline pathogenic or likely pathogenic (P/LP) variants were identified in 16 actionable genes and seven non-actionable genes according to French guidelines. 24/65 patients had previously received genetic analysis for diagnostic purposes, and for 17 of them, a P/LP variant had already been identified. Among the 48/65 remaining cases for which the EXOMA protocol revealed a previously unknown P/LP variant, only 19 met the criteria for genetic testing for inherited cancer risk after familial survey. These criteria had not been identified by the oncologist in 10 cases. In 21/65 cases, the variant was considered incidental.
Discussion: In 7.4% of patients, an undiagnosed hereditary genetic predisposition was identified, whether or not related to the clinical presentation, and germline analysis impacted oncological management for only 6.3% of the cohort. This low percentage should be weighed against the burden of systematic genetic consultation and urgent circuits. Information or training tools to form oncologists to the prescription of germline genetic analyses should be explored, as well as information supports and patient preferences.
Keywords: genetic counseling; incidental findings; information; oncogenetic; theranostic exome sequencing.
© 2023 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Gupta RG, Li F, Roszik J, Lizée G. Exploiting tumor neoantigens to target cancer evolution: current challenges and promising therapeutic approaches. Cancer Discov. 2021;11(5):1024‐1039. doi: 10.1158/2159-8290.CD-20-1575 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

