Genome Editing in Engineered T Cells for Cancer Immunotherapy
- PMID: 37694593
- PMCID: PMC10623081
- DOI: 10.1089/hum.2023.128
Genome Editing in Engineered T Cells for Cancer Immunotherapy
Abstract
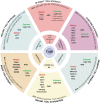
Advanced gene transfer technologies and profound immunological insights have enabled substantial increases in the efficacy of anticancer adoptive cellular therapy (ACT). In recent years, the U.S. Food and Drug Administration and European Medicines Agency have approved six engineered T cell therapeutic products, all chimeric antigen receptor-engineered T cells directed against B cell malignancies. Despite encouraging clinical results, engineered T cell therapy is still constrained by challenges, which could be addressed by genome editing. As RNA-guided Clustered Regularly Interspaced Short Palindromic Repeats technology passes its 10-year anniversary, we review emerging applications of genome editing approaches designed to (1) overcome resistance to therapy, including cancer immune evasion mechanisms; (2) avoid unwanted immune reactions related to allogeneic T cell products; (3) increase fitness, expansion capacity, persistence, and potency of engineered T cells, while preserving their safety profile; and (4) improve the ability of therapeutic cells to resist immunosuppressive signals active in the tumor microenvironment. Overall, these innovative approaches should widen the safe and effective use of ACT to larger number of patients affected by cancer.
Keywords: CAR; CRISPR; TCR.
Conflict of interest statement
C.B. is inventor on patent applications and has been granted patents related to T cell engineering that have been, in part, licensed to industry. C.B. has been member of Advisory Board and Consultant for Intellia Therapeutics, Novartis, GSK, Allogene, Kite/Gilead, Miltenyi, Kiadis, Evir, Janssen, Alia and received research support from Intellia Therapeutics.
A.G.C. is a scientific co-founder of Affini-T Therapeutics, is an inventor on patents related to TCRs, and has received funding from Affini-T, Amazon and Lonza.
S.G. is an inventor on patents related to CAR-T cell therapy, filed by the University of Pennsylvania and licensed to Novartis and Tmunity, and has received commercial research funding from Gilead.
C.F.M. is an inventor on patents related to CAR T and regulatory T cell therapy, filled by the Tettamanti Research Center (under license to Coimmune), and Yale University, San Raffaele Hospital and the Telethon Foundation.
M.H. is inventor on patent applications and has been granted patents related to CAR technology that have been, in part, licensed to industry. Co-founder and equity owner T-CURX GmbH, Würzburg, Germany. Funding: BMS. Speaker honoraria: Janssen, BMS, Novartis.
W.Q. is inventor in patents filed in relation to genome edited T cells; Funding- Servier, Cellectis, Miltenyi; Consultancy Wugen, Virocell, Skylark.
Figures
References
-
- Kolb HJ, Mittermüller J, Clemm C, et al. . Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood 1990;76:2462–2465. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

