Autism care pathway in Europe
- PMID: 37694810
- PMCID: PMC10594203
- DOI: 10.1192/j.eurpsy.2023.2435
Autism care pathway in Europe
Abstract
Background: Autism is a lifelong complex neurodevelopmental condition that affects brain development and behaviour with significant consequences for everyday life. Despite its personal, familial, and societal impact, Europe-wide harmonised guidelines are still lacking for early detection, diagnosis, and intervention, leading to an overall unsatisfactory autistic person and carer journey.
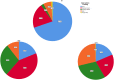
Methods: The care pathway for autistic children and adolescents was analysed in Italy, Spain and the UK from the perspective of carers (using a survey aimed at caregivers of autistic children 0-18 years old), the autistic community, and professionals in order to identify major barriers (treatment gaps) preventing carers from receiving information, support, and timely screening/diagnosis and intervention.
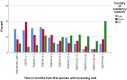
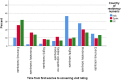
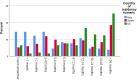
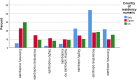
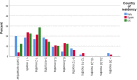
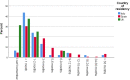
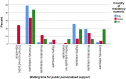
Results: Across all three countries, analysis of the current care pathway showed: long waits from the time carers raised their first concerns about a child's development and/or behaviour until screening and confirmed diagnosis; delayed or no access to intervention once a diagnosis was confirmed; limited information about autism and how to access early detection services; and deficient support for families throughout the journey.
Conclusions: These findings call for policy harmonisation in Europe to shorten long wait times for diagnosis and intervention and therefore, improve autistic people and their families' journey experience and quality of life.
Keywords: autism; diagnosis; early intervention; early screening; policy recommendations.
Conflict of interest statement
A.S.J.C. has received consultant fees from Roche and Servier. M.P. has been a consultant to or has received honoraria or grants from Otsuka, Exeltis, Janssen, Servier, and Exeltis. C.A. has been a consultant to or has received honoraria or grants from Acadia, Angelini, Biogen, Boehringer, Gedeon Richter, Janssen Cilag, Lundbeck, Medscape, Minerva, Otsuka, Pfizer, Roche, Sage, Servier, Shire, Schering Plough, Sumitomo Dainippon Pharma, Sunovion, and Takeda. D.G.M.M. has been a consultant for Roche, Servier, and Jaguar Therapeutics. R.C. has been a consultant for Servier. The other authors declare none.
Figures
References
-
- American Psychiatry Association. Diagnostic and statistical manual of mental disorders (DSM-5®). Arlington, VA: American Psychiatric Publications; 2013.
-
- Parellada M, Penzol MJ, Pina L, Moreno C, Gonzalez-Vioque E, Zalsman G, et al. The neurobiology of autism spectrum disorders. Eur Psychiatry. 2014;29(1):11–9. - PubMed
-
- World Health Organisation. International classification of diseases for mortality and morbidity statistics. 11th Revision. Geneva: WHO; 2018.
-
- Hill APZK, Fombonne E. Epidemiology of autism spectrum disorders. 4th ed. New York: Wiley & Sons; 2014.
-
- Baird G, Simonoff E, Pickles A, Chandler S, Loucas T, Meldrum D, et al. Prevalence of disorders of the autism spectrum in a population cohort of children in South Thames: The special needs and autism project (SNAP). Lancet. 2006;368(9531):210–5. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

