Efficacy and Tolerability of Gefapixant for Treatment of Refractory or Unexplained Chronic Cough: A Systematic Review and Dose-Response Meta-Analysis
- PMID: 37694849
- PMCID: PMC10495930
- DOI: 10.1001/jama.2023.18035
Efficacy and Tolerability of Gefapixant for Treatment of Refractory or Unexplained Chronic Cough: A Systematic Review and Dose-Response Meta-Analysis
Abstract
Importance: Gefapixant represents an emerging therapy for patients with refractory or unexplained chronic cough.
Objective: To evaluate the efficacy and tolerability of gefapixant for the treatment of adults with refractory or unexplained chronic cough.
Data sources: MEDLINE, Embase, Cochrane Central Register of Controlled Trials, and Web of Science from November 2014 to July 2023.
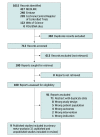
Study selection: Two reviewers independently screened for parallel and crossover randomized clinical trials (RCTs) that compared, in patients with refractory or unexplained chronic cough, either gefapixant with placebo, or 2 or more doses of gefapixant with or without placebo.
Data extraction and synthesis: Two reviewers independently extracted data. A frequentist random-effects dose-response meta-analysis or pairwise meta-analysis was used for each outcome. The GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) approach was used to rate the certainty in whether patients would perceive the effects as important (greater than the minimal important difference [MID]) or small (less than the MID).
Main outcomes and measures: Cough frequency (measured using the VitaloJAK cough monitor; MID, 20%), cough severity (measured using the 100-mm visual analog scale [VAS]; higher score is worse; MID, 30 mm), cough-specific quality of life (measured using the Leicester Cough Questionnaire [LCQ]; score range, 3 [maximal impairment] to 21 [no impairment]; MID, 1.3 points), treatment-related adverse events, adverse events leading to discontinuation, and taste-related adverse events.
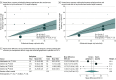
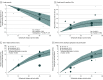
Results: Nine RCTs including 2980 patients were included in the primary analysis. Compared with placebo, gefapixant (45 mg twice daily) had small effects on awake cough frequency (17.6% reduction [95% CI, 10.6%-24.0%], moderate certainty), cough severity on the 100-mm VAS (mean difference, -6.2 mm [95% CI, -4.1 to -8.4]; high certainty), and cough-specific quality of life on the LCQ (mean difference, 1.0 points [95% CI, 0.7-1.4]; moderate certainty). Compared with placebo, gefapixant (45 mg twice daily) probably caused an important increase in treatment-related adverse events (32 more per 100 patients [95% CI, 13-64 more], moderate certainty) and taste-related adverse events (32 more per 100 patients [95% CI, 22-46 more], high certainty). High-certainty evidence suggests that gefapixant (15 mg twice daily) had small effects on taste-related adverse events (6 more per 100 patients [95% CI, 5-8 more]).
Conclusions and relevance: Compared with placebo, gefapixant (45 mg orally twice daily) led to modest improvements in cough frequency, cough severity, and cough-specific quality of life but increased taste-related adverse events.
Conflict of interest statement
Figures

Comment in
-
Gefapixant for Refractory or Unexplained Chronic Cough?JAMA. 2023 Oct 10;330(14):1335-1336. doi: 10.1001/jama.2023.18508. JAMA. 2023. PMID: 37694857 No abstract available.
-
Gefapixant for Chronic Cough.JAMA. 2024 Feb 13;331(6):529. doi: 10.1001/jama.2023.26032. JAMA. 2024. PMID: 38349378 No abstract available.
References
-
- French CT, Diekemper RL, Irwin RS, et al. ; CHEST Expert Cough Panel . Assessment of intervention fidelity and recommendations for researchers conducting studies on the diagnosis and treatment of chronic cough in the adult: CHEST guideline and expert panel report. Chest. 2015;148(1):32-54. doi: 10.1378/chest.15-0164 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

