Semisynthetic Pneumococcal Glycoconjugate Nanovaccine
- PMID: 37694903
- PMCID: PMC10515484
- DOI: 10.1021/acs.bioconjchem.3c00252
Semisynthetic Pneumococcal Glycoconjugate Nanovaccine
Abstract
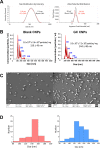
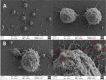
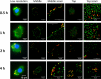
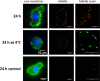
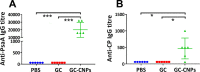
Pneumococcal conjugate vaccines offer an excellent safety profile and high protection against the serotypes comprised in the vaccine. However, inclusion of protein antigens fromStreptococcus pneumoniaecombined with potent adjuvants and a suitable delivery system are expected to both extend protection to serotype strains not represented in the formulation and stimulate a broader immune response, thus more effective in young children, elderly, and immunocompromised populations. Along this line, nanoparticle (NP) delivery systems can enhance the immunogenicity of antigens by protecting them from degradation and increasing their uptake by antigen-presenting cells, as well as offering co-delivery with adjuvants. We report herein the encapsulation of a semisynthetic glycoconjugate (GC) composed of a synthetic tetrasaccharide mimicking theS. pneumoniae serotype 14 capsular polysaccharide (CP14) linked to the Pneumococcal surface protein A (PsaA) using chitosan NPs (CNPs). These GC-loaded chitosan nanoparticles (GC-CNPs) were not toxic to human monocyte-derived dendritic cells (MoDCs), showed enhanced uptake, and displayed better immunostimulatory properties in comparison to the naked GC. A comparative study was carried out in mice to evaluate the immune response elicited by the glycoconjugate-administered subcutaneously (SC), where the GC-CNPs displayed 100-fold higher IgG response as compared with the group treated with nonencapsulated GC. Overall, the study demonstrates the potential of this chitosan-based nanovaccine for efficient delivery of glycoconjugate antigens.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Rapola S.; Jäntti V.; Haikala R.; Syrjänen R.; Carlone G. M.; Sampson J. S.; Briles D. E.; Paton J. C.; Takala A. K.; Kilpi T. M.; Käyhty H. Natural Development of Antibodies to Pneumococcal Surface Protein A, Pneumococcal Surface Adhesin A, and Pneumolysin in Relation to Pneumococcal Carriage and Acute Otitis Media. J. Infect. Dis. 2000, 182, 1146–1152. 10.1086/315822. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

