Expansion of plasma MicroRNAs over the first month following human stroke
- PMID: 37694957
- PMCID: PMC10925862
- DOI: 10.1177/0271678X231196982
Expansion of plasma MicroRNAs over the first month following human stroke
Abstract
Few have characterized miRNA expression during the transition from injury to neural repair and secondary neurodegeneration following stroke in humans. We compared expression of 754 miRNAs from plasma samples collected 5, 15, and 30 days post-ischemic stroke from a discovery cohort (n = 55) and 15-days post-ischemic stroke from a validation cohort (n = 48) to healthy control samples (n = 55 and 48 respectively) matched for age, sex, race and cardiovascular comorbidities using qRT-PCR. Eight miRNAs remained significantly altered across all time points in both cohorts including many described in acute stroke. The number of significantly dysregulated miRNAs more than doubled from post-stroke day 5 (19 miRNAs) to days 15 (50 miRNAs) and 30 (57 miRNAs). Twelve brain-enriched miRNAs were significantly altered at one or more time points (decreased expression, stroke versus controls: miR-107; increased expression: miR-99-5p, miR-127-3p, miR-128-3p, miR-181a-3p, miR-181a-5p, miR-382-5p, miR-433-3p, miR-491-5p, miR-495-3p, miR-874-3p, and miR-941). Many brain-enriched miRNAs were associated with apoptosis over the first month post-stroke whereas other miRNAs suggested a transition to synapse regulation and neuronal protection by day 30. These findings suggest that a program of decreased cellular proliferation may last at least 30 days post-stroke, and points to specific miRNAs that could contribute to neural repair in humans.
Keywords: Apoptosis; MicroRNAs; ischemic stroke; neuronal plasticity; neuroprotection.
Conflict of interest statement
Declaration of conflicting interestsThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures



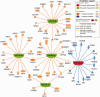

References
-
- Kosik KS. The neuronal microRNA system. Nat Rev Neurosci 2006; 7: 911–920. - PubMed
-
- Guarnieri DJ andDiLeone RJ.. MicroRNAs: a new class of gene regulators. Ann Med 2008; 40: 197–208. - PubMed
-
- Alvarez-Erviti L andSeow Y andYin H. et al.. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 2011; 29: 341–345. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

