A prospective, multicenter, observational study of ixazomib plus lenalidomide-dexamethasone in patients with relapsed/refractory multiple myeloma in Japan
- PMID: 37695378
- PMCID: PMC10798923
- DOI: 10.1007/s00277-023-05428-7
A prospective, multicenter, observational study of ixazomib plus lenalidomide-dexamethasone in patients with relapsed/refractory multiple myeloma in Japan
Abstract
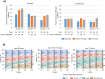
Real-world studies permit inclusion of a more diverse patient population and provide more information on the effectiveness of treatments used in routine clinical practice. This prospective, multicenter, observational study investigated the effectiveness and safety of ixazomib plus lenalidomide and dexamethasone (IRd) in 295 patients with relapsed/refractory multiple myeloma (RRMM) in routine clinical practice in Japan. Patients had a median age of 74 years, 80.0% were aged ≥ 65 years, 42.0% had received ≥ 3 lines of prior treatment, and 28.5% were "frail" according to the International Myeloma Working Group frailty score. After a median follow-up of 25.0 months, median progression-free survival (PFS) was 15.3 (95% CI 12.4-19.5) months, while median overall survival was not reached. The overall response rate was 53.9%, and 31.5% of patients had a very good partial response or better. In the subgroup analysis, median PFS was better in patients with 1 versus 2 or ≥ 3 lines of prior treatment (29.0 vs 19.2 or 6.9 months) and paraprotein versus clinical relapse (16.0 vs 7.9 months), but median PFS was not notably affected by frailty score or age group. Dose adjustment was more frequent among patients aged > 75 years, especially early after IRd treatment initiation. Treatment-emergent adverse events (TEAEs) of any grade occurred in 84.4% of patients and 24.7% of patients discontinued treatment due to TEAEs; no new safety concerns were found. These findings suggest that oral IRd triplet regimen is an effective and tolerable treatment option for RRMM patients in real-world settings outside of clinical trials.ClinicalTrials.gov identifier: NCT03433001; Date of registration: 14 February 2018.
Keywords: Effectiveness; Ixazomib; Multiple myeloma; Real-world data; Relapsed/refractory; Safety.
© 2023. The Author(s).
Conflict of interest statement
This study was approved by the institutional ethics committees of Nagoya City University Graduate School of Medical Sciences (Approval No.: 50–17-0002, 12 April 2018) and other participating institutes. This study was conducted in accordance with the ethical principles of the Declaration of Helsinki and the Ethical Guidelines for Medical and Health Research Involving Human Subjects, and complied with all applicable laws and regulations, including data privacy laws, and guidelines and regulations on conflicts of interest.
Yuichi Horigome has received honoraria for lectures and advisory board fees from Janssen. Takahiro Kobayashi has received speaker fees from Ono Pharmaceutical, Bristol-Myers Squibb, Takeda, Fujimoto, Janssen, and Sanofi. Hiroshi Handa has received research grants from Takeda, Bristol-Myers Squibb, and Kyowa Kirin; consulting fees from Takeda, Janssen, and Bristol-Myers Squibb; lecture fees from Takeda, Janssen, Bristol-Myers Squibb, Ono Pharmaceutical, and Sanofi; meeting fees, medical writing fees, and the article processing charge from Takeda; and counselling fees from Japanese Society of Myeloma. Masahiro Abe has received honoraria from Takeda, Bristol-Myers Squibb, Janssen, Sanofi, Ono Pharmaceutical, and Daiichi Sankyo and is the President of the Japanese Society of Myeloma. Tadao Ishida has received grants from Janssen and Bristol-Myers Squibb; and honoraria from Ono Pharmaceutical, Janssen, Bristol-Myers Squibb, Takeda, and Sanofi. Shigeki Ito has received clinical trial contracts from Bristol-Myers Squibb; and lecture fees from Bristol-Myers Squibb and Takeda. Hiromi Iwasaki has received grants from Kyowa Kirin; and honoraria from Takeda, Daiichi Sankyo, SymBio, LSI Medience, AbbVie, Ono Pharmaceutical, Chugai, Janssen, Sanofi, AstraZeneca, and Kyowa Kirin. Junya Kuroda has received grants from Kyowa Kirin, Chugai, Daiichi Sankyo, Ono Pharmaceutical, Eisai, Taiho, Sumitomo, Shionogi, and Bristol-Myers Squibb; honoraria from Ono Pharmaceutical, Sanofi, Bristol-Myers Squibb, and Janssen; advisory board fees from Janssen, Bristol-Myers Squibb, Asahikasei Pharma, and Pfizer; and fees for other services from Bristol-Myers Squibb and Taiho. Hirohiko Shibayama has received fees for manuscript writing and for attending meetings from Takeda; grants from Ono Pharmaceutical and Bristol-Myers Squibb; consulting fees from Takeda, Fujimoto, Eisai, AstraZeneca, Janssen, AbbVie, and Sanofi; and honoraria from Takeda, Chugai, AstraZeneca, Ono Pharmaceutical, Janssen, and Sanofi. Kazutaka Sunami has received research funding from Ono Pharmaceutical, Celgene, AbbVie, Takeda, Sanofi, Bristol-Myers Squibb, GlaxoSmithKline, Chugai, Otsuka, and Janssen; and honoraria from Ono Pharmaceutical, Bristol-Myers Squibb, Takeda, and Sanofi. Hiroyuki Takamatsu has received grants from Bristol-Myers Squibb; consulting fees from SRL; and honoraria from Janssen, Ono Pharmaceutical, Sanofi, and Bristol-Myers Squibb. Hideto Tamura has received lecture fees from Sanofi, Bristol-Myers Squibb, Ono Pharmaceutical, Janssen, Chugai, and Takeda. Toshiaki Hayashi has received speaker fees from Takeda, Bristol-Myers Squibb, Ono Pharmaceutical, Sanofi, and Fujimoto. Kiwamu Akagi has received lecture fees from Merck Sharp & Dohme, Chugai, AstraZeneca, Bristol-Myers Squibb, Mochida, Taiho, and Roche; and advisory board fees from Takeda. Takahiro Maeda has received grants from Japan Society for the Promotion of Science, the Japanese Society of Hematology, Kyowa Kirin, and Takeda; and honoraria from Kyowa Kirin, Otsuka, Novartis, Sumitomo, Chugai, Takeda, Astellas Pharma Inc., and AbbVie. Tomohiro Shinozaki has received lecture fees from Santen Pharmaceutical, Novartis, and CMIC Holdings Co., Ltd.; and fees for attending meetings from Takeda. Shinsuke Iida has received grants from Takeda, Sanofi, Janssen, AbbVie, Novartis, Amgen, GlaxoSmithKline, Bristol-Myers Squibb, Daiichi Sankyo, Ono Pharmaceutical, Chugai, Otsuka, Celgene, and Alexion; consulting fees from Takeda, Janssen, Pfizer, AbbVie, Sanofi, Otsuka, GlaxoSmithKline, Regeneron, Bristol-Myers Squibb, and Novartis; and lecture fees from Janssen, Takeda, Bristol-Myers Squibb, Sanofi, and Ono Pharmaceutical. Takahiro Yoshida and Ikuo Mori are employees of Takeda Pharmaceutical Co. Ltd. Masaki Iino, Yoriko Harazaki, Yasushi Hiramatsu, Taiga Kuroi, Kazuki Tanimoto, and Kosei Matsue have no conflicts of interest to declare.
Figures





References
-
- World Health Organization (2020) Global Cancer Observatory: Cancer Today (Japan). https://gco.iarc.fr/today/data/factsheets/populations/392-japan-fact-she.... Accessed 08 June 2022
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

