Two-Year Outcomes After Minimally Invasive Surfactant Therapy in Preterm Infants: Follow-Up of the OPTIMIST-A Randomized Clinical Trial
- PMID: 37695601
- PMCID: PMC10495923
- DOI: 10.1001/jama.2023.15694
Two-Year Outcomes After Minimally Invasive Surfactant Therapy in Preterm Infants: Follow-Up of the OPTIMIST-A Randomized Clinical Trial
Abstract
Importance: The long-term effects of surfactant administration via a thin catheter (minimally invasive surfactant therapy [MIST]) in preterm infants with respiratory distress syndrome remain to be definitively clarified.
Objective: To examine the effect of MIST on death or neurodevelopmental disability (NDD) at 2 years' corrected age.
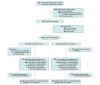
Design, setting, and participants: Follow-up study of a randomized clinical trial with blinding of clinicians and outcome assessors conducted in 33 tertiary-level neonatal intensive care units in 11 countries. The trial included 486 infants with a gestational age of 25 to 28 weeks supported with continuous positive airway pressure (CPAP). Collection of follow-up data at 2 years' corrected age was completed on December 9, 2022.
Interventions: Infants assigned to MIST (n = 242) received exogenous surfactant (200 mg/kg poractant alfa) via a thin catheter; those assigned to the control group (n = 244) received sham treatment.
Main outcomes and measures: The key secondary outcome of death or moderate to severe NDD was assessed at 2 years' corrected age. Other secondary outcomes included components of this composite outcome, as well as hospitalizations for respiratory illness and parent-reported wheezing or breathing difficulty in the first 2 years.
Results: Among the 486 infants randomized, 453 had follow-up data available (median gestation, 27.3 weeks; 228 females [50.3%]); data on the key secondary outcome were available in 434 infants. Death or NDD occurred in 78 infants (36.3%) in the MIST group and 79 (36.1%) in the control group (risk difference, 0% [95% CI, -7.6% to 7.7%]; relative risk [RR], 1.0 [95% CI, 0.81-1.24]); components of this outcome did not differ significantly between groups. Secondary respiratory outcomes favored the MIST group. Hospitalization with respiratory illness occurred in 49 infants (25.1%) in the MIST group vs 78 (38.2%) in the control group (RR, 0.66 [95% CI, 0.54-0.81]) and parent-reported wheezing or breathing difficulty in 73 (40.6%) vs 104 (53.6%), respectively (RR, 0.76 [95% CI, 0.63-0.90]).
Conclusions and relevance: In this follow-up study of a randomized clinical trial of preterm infants with respiratory distress syndrome supported with CPAP, MIST compared with sham treatment did not reduce the incidence of death or NDD by 2 years of age. However, infants who received MIST had lower rates of adverse respiratory outcomes during their first 2 years of life.
Trial registration: anzctr.org.au Identifier: ACTRN12611000916943.
Conflict of interest statement
Figures
Comment in
-
Outcomes After Minimally Invasive Surfactant Therapy in Preterm Infants.JAMA. 2024 Jan 23;331(4):360-361. doi: 10.1001/jama.2023.24530. JAMA. 2024. PMID: 38261051 No abstract available.
-
Outcomes After Minimally Invasive Surfactant Therapy in Preterm Infants.JAMA. 2024 Jan 23;331(4):360. doi: 10.1001/jama.2023.24527. JAMA. 2024. PMID: 38261052 No abstract available.
-
EBNEO Commentary: De-MIST-ifying the 2-year outcomes of non-invasive surfactant therapy.Acta Paediatr. 2024 May;113(5):1121-1122. doi: 10.1111/apa.17116. Epub 2024 Jan 25. Acta Paediatr. 2024. PMID: 38269640 No abstract available.

