Cu-Catalyzed Enantioselective Protoboration of 2,3-Disubstituted 1,3-Dienes
- PMID: 37695719
- PMCID: PMC10521025
- DOI: 10.1021/acs.orglett.3c02627
Cu-Catalyzed Enantioselective Protoboration of 2,3-Disubstituted 1,3-Dienes
Abstract
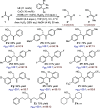
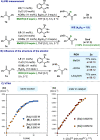
A Cu-catalyzed regio- and enantioselective protoboration of 2,3-disubstituted 1,3-dienes is described. The protocol operates under mild conditions and is applicable to symmetrically and unsymmetrically substituted dienes, providing access to homoallylic boronates in consistently high yield, regioselectivity, and enantiomeric ratio. Preliminary investigations point to a complex mechanism.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Semba K.; Fujihara T.; Terao J.; Tsuji Y. Copper-Catalyzed Borylative Transformations of Non-Polar Carbon–Carbon Unsaturated Compounds Employing Borylcopper as an Active Catalyst Species. Tetrahedron 2015, 71, 2183–2197. 10.1016/j.tet.2015.02.027. - DOI
- Perry G. J. P.; Jia T.; Procter D. J. Copper-Catalyzed Functionalization of 1,3-Dienes: Hydrofunctionalization, Borofunctionalization, and Difunctionalization. ACS Catal. 2020, 10, 1485–1499. 10.1021/acscatal.9b04767. - DOI
- Whyte A.; Torelli A.; Mirabi B.; Zhang A.; Lautens M. Copper-Catalyzed Borylative Difunctionalization of π-Systems. ACS Catal. 2020, 10, 11578–11622. 10.1021/acscatal.0c02758. - DOI
-
- Sasaki Y.; Zhong C.; Sawamura M.; Ito H. Copper (I)-Catalyzed Asymmetric Monoborylation of 1,3-Dienes: Synthesis of Enantioenriched Cyclic Homoallyl-and Allylboronates. J. Am. Chem. Soc. 2010, 132, 1226–1227. 10.1021/ja909640b. - DOI - PubMed
- Liu Y.; Fiorito D.; Mazet C. Copper-Catalyzed Enantioselective 1,2-Borylation of 1,3-Dienes. Chem. Sci. 2018, 9, 5284–5288. 10.1039/C8SC01538D. - DOI - PMC - PubMed
- Guan Q.; Ji Y.; Zhao Q.; Zhang C. Copper-Catalyzed Highly Enantioselective 1,4-Protoboration of Terminal 1,3-Dienes. CCS Chem. 2022, 4, 1545–1556. 10.31635/ccschem.021.202100947. - DOI
- Xu R. S.; Rohde L. N. Jr.; Diver S. T. Regioselective Cu-Catalyzed Hydroboration of 1,3-Disubstituted-1,3-Dienes: Functionalization of Conjugated Dienes Readily Accessible through Ene-Yne Metathesis. ACS Catal. 2022, 12, 6434–6443. 10.1021/acscatal.2c01190. - DOI
-
- Fehr C. Enantioselective Protonation of Enolates and Enols. Angew. Chem., Int. Ed. 1996, 35, 2566–2587. 10.1002/anie.199625661. - DOI
- Mohr J. T.; Hong A. Y.; Stoltz B. M. Enantioselective Protonation. Nat. Chem. 2009, 1, 359–369. 10.1038/nchem.297. - DOI - PMC - PubMed
- Oudeyer S.; Briere J.-F.; Levacher V. Progress in Catalytic Asymmetric Protonation. Eur. J. Org. Chem. 2014, 2014, 6103–6119. 10.1002/ejoc.201402213. - DOI
-
- Palais L.; Mikhel I. S.; Bournaud C.; Micouin L.; Falciola C. A.; Augustin M. V.; Rosset S.; Bernardinelli G.; Alexakis A. SimplePhos Monodentate Ligands: Synthesis and Application in Copper-Catalyzed Reactions. Angew. Chem., Int. Ed. 2007, 119, 7606–7609. 10.1002/ange.200702186. - DOI - PubMed
- Fiorito D.; Liu Y. B.; Besnard C.; Mazet C. Direct Access to Chiral Secondary Amides by Copper-Catalyzed Borylative Carboxamidation of Vinylarenes with Isocyanates. J. Am. Chem. Soc. 2020, 142, 623–632. 10.1021/jacs.9b12297. - DOI - PubMed
-
- Jarava-Barrera C.; Parra A.; López A.; Cruz-Acosta F.; Collado-Sanz D.; Cárdenas D. J.; Tortosa M. Copper-Catalyzed Borylative Aromatization of p-Quinone Methides: Enantioselective Synthesis of Dibenzylic Boronates. ACS Catal. 2016, 6, 442–446. 10.1021/acscatal.5b02742. - DOI - PMC - PubMed
- Kubota K.; Watanabe Y.; Hayama K.; Ito H. Enantioselective Synthesis of Chiral Piperidines via the Stepwise Dearomatization/Borylation of Pyridines. J. Am. Chem. Soc. 2016, 138, 4338–4341. 10.1021/jacs.6b01375. - DOI - PubMed
- Desfeux C.; Besnard C.; Mazet C. [n]Dendralenes as a Platform for Selective Catalysis: Ligand-Controlled Cu-Catalyzed Chemo-, Regio-, and Enantioselective Borylations. Org. Lett. 2020, 22, 8181–8187. 10.1021/acs.orglett.0c01892. - DOI - PubMed
- Wu J.; Wu H.; Li X.; Liu X.; Zhao Q.; Huang G.; Zhang C. Copper-Catalyzed Highly Selective Protoboration of CF3-Containing 1,3-Dienes. Angew. Chem., Int. Ed. 2021, 133, 20539–20545. 10.1002/ange.202105896. - DOI - PubMed
LinkOut - more resources
Full Text Sources

