Antimalarial Dibenzannulated Medium-Ring Keto Lactams
- PMID: 37695781
- PMCID: PMC10860121
- DOI: 10.1021/acsinfecdis.3c00245
Antimalarial Dibenzannulated Medium-Ring Keto Lactams
Abstract
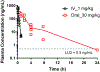
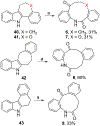
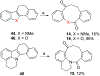
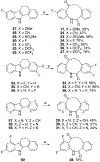
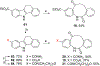
We discovered dibenzannulated medium-ring keto lactams (11,12-dihydro-5H-dibenzo[b,g]azonine-6,13-diones) as a new antimalarial chemotype. Most of these had chromatographic LogD7.4 values ranging from <0 to 3 and good kinetic solubilities (12.5 to >100 μg/mL at pH 6.5). The more polar compounds in the series (LogD7.4 values of <2) had the best metabolic stability (CLint values of <50 μL/min/mg protein in human liver microsomes). Most of the compounds had relatively low cytotoxicity, with IC50 values >30 μM, and there was no correlation between antiplasmodial activity and cytotoxicity. The four most potent compounds had Plasmodium falciparum IC50 values of 4.2 to 9.4 nM and in vitro selectivity indices of 670 to >12,000. They were more than 4 orders-of-magnitude less potent against three other protozoal pathogens (Trypanosoma brucei rhodesiense, Trypanosoma cruzi, and Leishmania donovani) but did have relatively high potency against Toxoplasma gondii, with IC50 values ranging from 80 to 200 nM. These keto lactams are converted into their poorly soluble 4(1H)-quinolone transannular condensation products in vitro in culture medium and in vivo in mouse blood. The similar antiplasmodial potencies of three keto lactam-quinolone pairs suggest that the quinolones likely contribute to the antimalarial activity of the lactams.
Keywords: SAR; antimalarial; lactams; medium rings; quinolones.
Figures
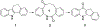


References
-
- Ashton TD; Devine SM; Möhrle JJ; Laleu B; Burrows JN; Charman SA; Creek DJ; Sleebs BE The development process for discovery and clinical advancement of modern antimalarials. J. Med. Chem 2019, 62, 10526–10562. - PubMed
-
- Witkop B; Patrick JB; Rosenblum M Ring effects in autoxidation. A new type of Camps reaction. J. Am. Chem. Soc 1951, 73, 2641–2647.
-
- Mentel M; Breinbauer R The Witkop-Winterfeldt oxidation of indoles. Curr. Org. Chem 2007, 11, 159–176.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

