Inflammatory Activity of Epithelial Stem Cell Variants from Cystic Fibrosis Lungs Is Not Resolved by CFTR Modulators
- PMID: 37695863
- PMCID: PMC10870857
- DOI: 10.1164/rccm.202305-0818OC
Inflammatory Activity of Epithelial Stem Cell Variants from Cystic Fibrosis Lungs Is Not Resolved by CFTR Modulators
Erratum in
-
Erratum: Inflammatory Activity of Epithelial Stem Cell Variants from Cystic Fibrosis Lungs Is Not Resolved by CFTR Modulators.Am J Respir Crit Care Med. 2024 Jul 15;210(2):249. doi: 10.1164/rccm.v209erratum10. Am J Respir Crit Care Med. 2024. PMID: 39007621 Free PMC article. No abstract available.
Abstract
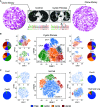
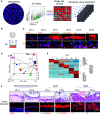
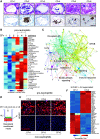
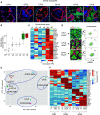
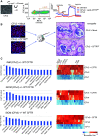
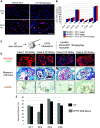
Rationale: CFTR (cystic fibrosis transmembrane conductance regulator) modulator drugs restore function to mutant channels in patients with cystic fibrosis (CF) and lead to improvements in body mass index and lung function. Although it is anticipated that early childhood treatment with CFTR modulators will significantly delay or even prevent the onset of advanced lung disease, lung neutrophils and inflammatory cytokines remain high in patients with CF with established lung disease despite modulator therapy, underscoring the need to identify and ultimately target the sources of this inflammation in CF lungs. Objectives: To determine whether CF lungs, like chronic obstructive pulmonary disease (COPD) lungs, harbor potentially pathogenic stem cell "variants" distinct from the normal p63/Krt5 lung stem cells devoted to alveolar fates, to identify specific variants that might contribute to the inflammatory state of CF lungs, and to assess the impact of CFTR genetic complementation or CFTR modulators on the inflammatory variants identified herein. Methods: Stem cell cloning technology developed to resolve pathogenic stem cell heterogeneity in COPD and idiopathic pulmonary fibrosis lungs was applied to end-stage lungs of patients with CF (three homozygous CFTR:F508D, one CFTR F508D/L1254X; FEV1, 14-30%) undergoing therapeutic lung transplantation. Single-cell-derived clones corresponding to the six stem cell clusters resolved by single-cell RNA sequencing of these libraries were assessed by RNA sequencing and xenografting to monitor inflammation, fibrosis, and mucin secretion. The impact of CFTR activity on these variants after CFTR gene complementation or exposure to CFTR modulators was assessed by molecular and functional studies. Measurements and Main Results: End-stage CF lungs display a stem cell heterogeneity marked by five predominant variants in addition to the normal lung stem cell, of which three are proinflammatory both at the level of gene expression and their ability to drive neutrophilic inflammation in xenografts in immunodeficient mice. The proinflammatory functions of these three variants were unallayed by genetic or pharmacological restoration of CFTR activity. Conclusions: The emergence of three proinflammatory stem cell variants in CF lungs may contribute to the persistence of lung inflammation in patients with CF with advanced disease undergoing CFTR modulator therapy.
Keywords: CFTR modulators; cystic fibrosis; lung inflammation; lung stem cells; neutrophils..
Figures

Comment in
-
Cystic Fibrosis Airways: Does Disease Stem from Faulty Stem Cells?Am J Respir Crit Care Med. 2023 Nov 1;208(9):913-914. doi: 10.1164/rccm.202309-1669ED. Am J Respir Crit Care Med. 2023. PMID: 37751560 Free PMC article. No abstract available.
-
Phoenix from the Ashes: Celebrating the 2023 North American Cystic Fibrosis Conference.Am J Respir Crit Care Med. 2023 Nov 1;208(9):909-910. doi: 10.1164/rccm.202309-1603ED. Am J Respir Crit Care Med. 2023. PMID: 37756479 Free PMC article. No abstract available.
References
-
- Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med . 2005;352:1992–2001. - PubMed
-
- Elborn JS. Cystic fibrosis. Lancet . 2016;388:2519–2531. - PubMed
-
- Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science . 1989;245:1066–1073. - PubMed
-
- Matsui H, Grubb BR, Tarran R, Randell SH, Gatzy JT, Davis CW, et al. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell . 1998;95:1005–1015. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA272906/CA/NCI NIH HHS/United States
- R01 HL136961/HL/NHLBI NIH HHS/United States
- R01 HL149678/HL/NHLBI NIH HHS/United States
- U24 CA228550/CA/NCI NIH HHS/United States
- R24 RR015088/RR/NCRR NIH HHS/United States
- R01 HL157100/HL/NHLBI NIH HHS/United States
- P30 DK054759/DK/NIDDK NIH HHS/United States
- P01 HL108808/HL/NHLBI NIH HHS/United States
- R01 DK115445/DK/NIDDK NIH HHS/United States
- P30 ES005605/ES/NIEHS NIH HHS/United States
- R01 HL136370/HL/NHLBI NIH HHS/United States
- R01 HL138510/HL/NHLBI NIH HHS/United States
- R01 HL165404/HL/NHLBI NIH HHS/United States
- R01 HL129795/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

