Animal FAS-like polyketide synthases produce diverse polypropionates
- PMID: 37695909
- PMCID: PMC10515154
- DOI: 10.1073/pnas.2305575120
Animal FAS-like polyketide synthases produce diverse polypropionates
Abstract
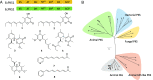
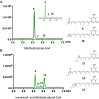
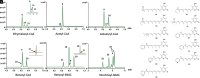
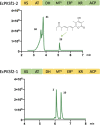
Animal cytoplasmic fatty acid synthase (FAS) represents a unique family of enzymes that are classically thought to be most closely related to fungal polyketide synthase (PKS). Recently, a widespread family of animal lipid metabolic enzymes has been described that bridges the gap between these two ubiquitous and important enzyme classes: the animal FAS-like PKSs (AFPKs). Although very similar in sequence to FAS enzymes that produce saturated lipids widely found in animals, AFPKs instead produce structurally diverse compounds that resemble bioactive polyketides. Little is known about the factors that bridge lipid and polyketide synthesis in the animals. Here, we describe the function of EcPKS2 from Elysia chlorotica, which synthesizes a complex polypropionate natural product found in this mollusc. EcPKS2 starter unit promiscuity potentially explains the high diversity of polyketides found in and among molluscan species. Biochemical comparison of EcPKS2 with the previously described EcPKS1 reveals molecular principles governing substrate selectivity that should apply to related enzymes encoded within the genomes of photosynthetic gastropods. Hybridization experiments combining EcPKS1 and EcPKS2 demonstrate the interactions between the ketoreductase and ketosynthase domains in governing the product outcomes. Overall, these findings enable an understanding of the molecular principles of structural diversity underlying the many molluscan polyketides likely produced by the diverse AFPK enzyme family.
Keywords: metazoan biosynthesis; polyketide synthase; sacoglossan polypropionate.
Conflict of interest statement
The authors declare no competing interest.
Figures


Similar articles
-
The polyketide to fatty acid transition in the evolution of animal lipid metabolism.Nat Commun. 2024 Jan 3;15(1):236. doi: 10.1038/s41467-023-44497-0. Nat Commun. 2024. PMID: 38172109 Free PMC article.
-
Animal biosynthesis of complex polyketides in a photosynthetic partnership.Nat Commun. 2020 Jun 8;11(1):2882. doi: 10.1038/s41467-020-16376-5. Nat Commun. 2020. PMID: 32513940 Free PMC article.
-
The Structural Enzymology of Iterative Aromatic Polyketide Synthases: A Critical Comparison with Fatty Acid Synthases.Annu Rev Biochem. 2018 Jun 20;87:503-531. doi: 10.1146/annurev-biochem-063011-164509. Annu Rev Biochem. 2018. PMID: 29925265 Review.
-
Metagenomic data reveals type I polyketide synthase distributions across biomes.mSystems. 2023 Jun 29;8(3):e0001223. doi: 10.1128/msystems.00012-23. Epub 2023 Jun 5. mSystems. 2023. PMID: 37272717 Free PMC article.
-
Iterative polyketide biosynthesis by modular polyketide synthases in bacteria.Appl Microbiol Biotechnol. 2016 Jan;100(2):541-57. doi: 10.1007/s00253-015-7093-0. Epub 2015 Nov 9. Appl Microbiol Biotechnol. 2016. PMID: 26549236 Free PMC article. Review.
Cited by
-
Phylogenetic systematics of the genus Cyerce (Mollusca: Heterobranchia: Sacoglossa: Caliphyllidae) from the Pacific and Indian oceans with descriptions of nine new species.Zool J Linn Soc. 2025 May 17;204(1):zlaf030. doi: 10.1093/zoolinnean/zlaf030. eCollection 2025 May. Zool J Linn Soc. 2025. PMID: 40386083
-
The structure of full-length AFPK supports the ACP linker in a role that regulates iterative polyketide and fatty acid assembly.Proc Natl Acad Sci U S A. 2025 Feb 11;122(6):e2419884122. doi: 10.1073/pnas.2419884122. Epub 2025 Feb 6. Proc Natl Acad Sci U S A. 2025. PMID: 39913209 Free PMC article.
-
Defensive polyketides produced by an abundant gastropod are candidate keystone molecules in estuarine ecology.Sci Adv. 2024 Nov;10(44):eadp8643. doi: 10.1126/sciadv.adp8643. Epub 2024 Oct 30. Sci Adv. 2024. PMID: 39475615 Free PMC article.
-
Chromosome-level genome assembly of the sacoglossan sea slug Elysia timida (Risso, 1818).BMC Genomics. 2024 Oct 7;25(1):941. doi: 10.1186/s12864-024-10829-7. BMC Genomics. 2024. PMID: 39375624 Free PMC article.
-
Synthetic Biology in Natural Product Biosynthesis.Chem Rev. 2025 Apr 9;125(7):3814-3931. doi: 10.1021/acs.chemrev.4c00567. Epub 2025 Mar 21. Chem Rev. 2025. PMID: 40116601 Review.
References
-
- Walsh C. T., Tang Y., Natural Product Biosynthesis (The Royal Society of Chemistry, 2017).
-
- Hertweck C., The biosynthetic logic of polyketide diversity. Angew. Chem. Int. Ed. 48, 4688–4716 (2009). - PubMed
-
- Rittner A., Paithankar K. S., Huu K. V., Grininger M., Characterization of the polyspecific transferase of murine type I fatty acid synthase (FAS) and implications for polyketide synthase (PKS) engineering. ACS Chem. Biol. 13, 723–732 (2018). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

