Serine starvation silences estrogen receptor signaling through histone hypoacetylation
- PMID: 37695911
- PMCID: PMC10515173
- DOI: 10.1073/pnas.2302489120
Serine starvation silences estrogen receptor signaling through histone hypoacetylation
Abstract
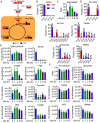
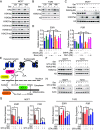
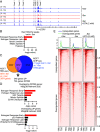
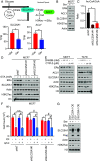
Loss of estrogen receptor (ER) pathway activity promotes breast cancer progression, yet how this occurs remains poorly understood. Here, we show that serine starvation, a metabolic stress often found in breast cancer, represses estrogen receptor alpha (ERα) signaling by reprogramming glucose metabolism and epigenetics. Using isotope tracing and time-resolved metabolomic analyses, we demonstrate that serine is required to maintain glucose flux through glycolysis and the TCA cycle to support acetyl-CoA generation for histone acetylation. Consequently, limiting serine depletes histone H3 lysine 27 acetylation (H3K27ac), particularly at the promoter region of ER pathway genes including the gene encoding ERα, ESR1. Mechanistically, serine starvation impairs acetyl-CoA-dependent gene expression by inhibiting the entry of glycolytic carbon into the TCA cycle and down-regulating the mitochondrial citrate exporter SLC25A1, a critical enzyme in the production of nucleocytosolic acetyl-CoA from glucose. Consistent with this model, total H3K27ac and ERα expression are suppressed by SLC25A1 inhibition and restored by acetate, an alternate source of acetyl-CoA, in serine-free conditions. We thus uncover an unexpected role for serine in sustaining ER signaling through the regulation of acetyl-CoA metabolism.
Keywords: SLC25A1; breast cancer; estrogen receptor; histone acetylation; serine metabolism.
Conflict of interest statement
The authors declare no competing interest.
Figures

References
-
- Couse J. F., Korach K. S., Estrogen receptor null mice: What have we learned and where will they lead us? Endocr. Rev. 20, 358–417 (1999). - PubMed
-
- Lim E., et al. , Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat. Med. 15, 907–913 (2009). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

