Effect of Metformin Versus Placebo on New Primary Cancers in Canadian Cancer Trials Group MA.32: A Secondary Analysis of a Phase III Randomized Double-Blind Trial in Early Breast Cancer
- PMID: 37695982
- PMCID: PMC10713140
- DOI: 10.1200/JCO.23.00296
Effect of Metformin Versus Placebo on New Primary Cancers in Canadian Cancer Trials Group MA.32: A Secondary Analysis of a Phase III Randomized Double-Blind Trial in Early Breast Cancer
Abstract
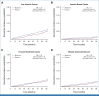
Clinical trials frequently include multiple end points that mature at different times. The initial report, typically based on the primary end point, may be published when key planned coprimary or secondary analyses are not yet available. Clinical trial updates provide an opportunity to disseminate additional results from studies, published in JCO or elsewhere, for which the primary end point has already been reported.Metformin has been associated with lower cancer risk in epidemiologic and preclinical research. In the MA.32 randomized adjuvant breast cancer trial, metformin (v placebo) did not affect invasive disease-free or overall survival. Here, we report metformin effects on the risk of new cancer. Between 2010 and 2013, 3,649 patients with breast cancer younger than 75 years without diabetes with high-risk T1-3, N0-3 M0 breast cancer (any estrogen receptor, progesterone receptor, human epidermal growth factor receptor 2) were randomly assigned to metformin 850 mg orally twice a day or placebo twice a day for 5 years. New primary invasive cancers (outside the ipsilateral breast) developing as a first event were identified. Time to events was described by the competing risks method; two-sided likelihood ratio tests adjusting for age, BMI, smoking, and alcohol intake were used to compare metformin versus placebo arms. A total of 184 patients developed new invasive cancers: 102 metformin and 82 placebo, hazard ratio (HR), 1.25; 95% CI, 0.94 to 1.68; P = .13. These included 48 contralateral invasive breast cancers (27 metformin v 21 placebo), HR, 1.29; 95% CI, 0.72 to 2.27; P = .40 and 136 new nonbreast primary cancers (75 metformin v 61 placebo), HR, 1.24; 95% CI, 0.88 to 1.74; P = .21. Metformin did not reduce the risk of new cancer development in these nondiabetic patients with breast cancer.
Trial registration: ClinicalTrials.gov NCT01101438.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures
References
-
- Zhang K, Bai P, Dai H, et al. : Metformin and risk of cancer among patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Prim Care Diabetes 15:52-58, 2021 - PubMed
-
- Tang GH, Satkunam M, Pond G, et al. : Association of metformin with breast cancer incidence and mortality in patients with type II diabetes: A GRADE-assessed systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 27:627-635, 2018 - PubMed
-
- Hevroni G, Skwiersky S, Zhyvotovska A, et al. : Metformin use and the risk of gastrointestinal malignancies in diabetic populations: A meta-analysis. Int J Clin Endocronol Metab 6:035-041, 2020
-
- Ng C-AW, Jiang AA, Toh EMS, et al. : Metformin and colorectal cancer: A systematic review, meta-analysis and meta-regression. Int J Colorectal Dis 35:1501-1512, 2020 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

