Pharmacological and electronic cigarette interventions for smoking cessation in adults: component network meta-analyses
- PMID: 37696529
- PMCID: PMC10495240
- DOI: 10.1002/14651858.CD015226.pub2
Pharmacological and electronic cigarette interventions for smoking cessation in adults: component network meta-analyses
Abstract
Background: Tobacco smoking is the leading preventable cause of death and disease worldwide. Stopping smoking can reduce this harm and many people would like to stop. There are a number of medicines licenced to help people quit globally, and e-cigarettes are used for this purpose in many countries. Typically treatments work by reducing cravings to smoke, thus aiding initial abstinence and preventing relapse. More information on comparative effects of these treatments is needed to inform treatment decisions and policies.
Objectives: To investigate the comparative benefits, harms and tolerability of different smoking cessation pharmacotherapies and e-cigarettes, when used to help people stop smoking tobacco.
Search methods: We identified studies from recent updates of Cochrane Reviews investigating our interventions of interest. We updated the searches for each review using the Cochrane Tobacco Addiction Group (TAG) specialised register to 29 April 2022.
Selection criteria: We included randomised controlled trials (RCTs), cluster-RCTs and factorial RCTs, which measured smoking cessation at six months or longer, recruited adults who smoked combustible cigarettes at enrolment (excluding pregnant people) and randomised them to approved pharmacotherapies and technologies used for smoking cessation worldwide (varenicline, cytisine, nortriptyline, bupropion, nicotine replacement therapy (NRT) and e-cigarettes) versus no pharmacological intervention, placebo (control) or another approved pharmacotherapy. Studies providing co-interventions (e.g. behavioural support) were eligible if the co-intervention was provided equally to study arms.
Data collection and analysis: We followed standard Cochrane methods for screening, data extraction and risk of bias (RoB) assessment (using the RoB 1 tool). Primary outcome measures were smoking cessation at six months or longer, and the number of people reporting serious adverse events (SAEs). We also measured withdrawals due to treatment. We used Bayesian component network meta-analyses (cNMA) to examine intervention type, delivery mode, dose, duration, timing in relation to quit day and tapering of nicotine dose, using odds ratios (OR) and 95% credibility intervals (CrIs). We calculated an effect estimate for combination NRT using an additive model. We evaluated the influence of population and study characteristics, provision of behavioural support and control arm rates using meta-regression. We evaluated certainty using GRADE.
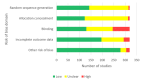
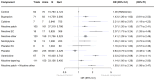
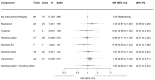
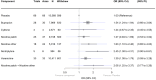
Main results: Of our 332 eligible RCTs, 319 (835 study arms, 157,179 participants) provided sufficient data to be included in our cNMA. Of these, we judged 51 to be at low risk of bias overall, 104 at high risk and 164 at unclear risk, and 118 reported pharmaceutical or e-cigarette/tobacco industry funding. Removing studies at high risk of bias did not change our interpretation of the results. Benefits We found high-certainty evidence that nicotine e-cigarettes (OR 2.37, 95% CrI 1.73 to 3.24; 16 RCTs, 3828 participants), varenicline (OR 2.33, 95% CrI 2.02 to 2.68; 67 RCTs, 16,430 participants) and cytisine (OR 2.21, 95% CrI 1.66 to 2.97; 7 RCTs, 3848 participants) were associated with higher quit rates than control. In absolute terms, this might lead to an additional eight (95% CrI 4 to 13), eight (95% CrI 6 to 10) and seven additional quitters per 100 (95% CrI 4 to 12), respectively. These interventions appeared to be more effective than the other interventions apart from combination NRT (patch and a fast-acting form of NRT), which had a lower point estimate (calculated additive effect) but overlapping 95% CrIs (OR 1.93, 95% CrI 1.61 to 2.34). There was also high-certainty evidence that nicotine patch alone (OR 1.37, 95% CrI 1.20 to 1.56; 105 RCTs, 37,319 participants), fast-acting NRT alone (OR 1.41, 95% CrI 1.29 to 1.55; 120 RCTs, 31,756 participants) and bupropion (OR 1.43, 95% CrI 1.26 to 1.62; 71 RCTs, 14,759 participants) were more effective than control, resulting in two (95% CrI 1 to 3), three (95% CrI 2 to 3) and three (95% CrI 2 to 4) additional quitters per 100 respectively. Nortriptyline is probably associated with higher quit rates than control (OR 1.35, 95% CrI 1.02 to 1.81; 10 RCTs, 1290 participants; moderate-certainty evidence), resulting in two (CrI 0 to 5) additional quitters per 100. Non-nicotine/placebo e-cigarettes (OR 1.16, 95% CrI 0.74 to 1.80; 8 RCTs, 1094 participants; low-certainty evidence), equating to one additional quitter (95% CrI -2 to 5), had point estimates favouring the intervention over control, but CrIs encompassed the potential for no difference and harm. There was low-certainty evidence that tapering the dose of NRT prior to stopping treatment may improve effectiveness; however, 95% CrIs also incorporated the null (OR 1.14, 95% CrI 1.00 to 1.29; 111 RCTs, 33,156 participants). This might lead to an additional one quitter per 100 (95% CrI 0 to 2). Harms There were insufficient data to include nortriptyline and non-nicotine EC in the final SAE model. Overall rates of SAEs for the remaining treatments were low (average 3%). Low-certainty evidence did not show a clear difference in the number of people reporting SAEs for nicotine e-cigarettes, varenicline, cytisine or NRT when compared to no pharmacotherapy/e-cigarettes or placebo. Bupropion may slightly increase rates of SAEs, although the CrI also incorporated no difference (moderate certainty). In absolute terms bupropion may cause one more person in 100 to experience an SAE (95% CrI 0 to 2).
Authors' conclusions: The most effective interventions were nicotine e-cigarettes, varenicline and cytisine (all high certainty), as well as combination NRT (additive effect, certainty not rated). There was also high-certainty evidence for the effectiveness of nicotine patch, fast-acting NRT and bupropion. Less certain evidence of benefit was present for nortriptyline (moderate certainty), non-nicotine e-cigarettes and tapering of nicotine dose (both low certainty). There was moderate-certainty evidence that bupropion may slightly increase the frequency of SAEs, although there was also the possibility of no increased risk. There was no clear evidence that any other tested interventions increased SAEs. Overall, SAE data were sparse with very low numbers of SAEs, and so further evidence may change our interpretation and certainty. Future studies should report SAEs to strengthen certainty in this outcome. More head-to-head comparisons of the most effective interventions are needed, as are tests of combinations of these. Future work should unify data from behavioural and pharmacological interventions to inform approaches to combined support for smoking cessation.
Copyright © 2023 The Authors. Cochrane Database of Systematic Reviews published by John Wiley & Sons, Ltd. on behalf of The Cochrane Collaboration.
Conflict of interest statement
NL was employed by the University of Oxford to work as the Managing Editor for the Cochrane Tobacco Addiction Review Group during this project; she was not involved in the editorial process for this review. Core infrastructure funding for the Group was provided by the National Institute of Health Research (NIHR) to the University of Oxford. Currently, NL is employed by the Oxford University Hospitals NHS Foundation Trust to act as an Associate Lecturer for Cochrane UK, teaching Cochrane authors how to write a Cochrane protocol. NL declares grants to carry out research (systematic reviews, RCTs and priority setting projects) from Cancer Research UK and the NIHR (a part of the NHS), both of whom have interests in people stopping smoking and run educational campaigns and in the latter case provide treatment to encourage people to stop smoking. NL also receives funding from Clarion Futures to carry out a service evaluation of smoking cessation support (i.e. very brief advice to quit smoking) offered as part of a financial advice service. In all aforementioned cases, payments have been made either to the University of Birmingham or the University of Oxford; however, NL has benefitted in the form of a salary. NL has written pieces for The Conversation on the findings of Cochrane Reviews assessing the effects of treatments for smoking cessation; these are evidence‐based and not based on personal opinion. NL was the Trial Manager of a study (a clinical trial of nicotine preloading run by the University of Birmingham/University of Oxford and funded by the NIHR HTA) eligible for inclusion in the work and was involved in all aspects of that study; the funder (NIHR) had no role in the interpretation of the findings of that study or this review. NL was not involved in the screening, data extraction or risk of bias assessment for this study. NL was involved in GRADE ratings for comparisons that this study was included in; however, the comparisons being assessed in this review were not the same as the comparison under investigation in the trial (i.e. nicotine preloading).
AT: none.
JMOM: none.
TRF receives funding from the NIHR Community Healthcare MedTech and In Vitro Diagnostics Co‐operative at Oxford Health NHS Foundation Trust (MIC‐2016‐018) and the NIHR Applied Research Collaboration Oxford and Thames Valley at Oxford Health NHS Foundation Trust. These funds are all paid to the University of Oxford; however, TRF benefits in the form of a salary.
AJS carries out consultancy for GlaxoSmithKline; however, this does not relate to any of the products investigated in this review or any products indicated for smoking cessation; personal payments. AJS is named on the NIHR Applied Research Collaboration East Midlands and Leicester NIHR Biomedical Research Centre (BRC) grants and is the grant holder for the NIHR Complex Reviews Support Unit (project number 14/178/29); all paid to the University of Leicester.
JLB was employed by the University of Oxford to work as a Managing Editor and Information Specialist for the Cochrane Tobacco Addiction Review Group during this project. He is now an Editor for Cochrane. In all cases, he was not involved in the editorial process for this review. Core infrastructure funding for the Cochrane Tobacco Addiction Group was provided by the NIHR to the University of Oxford.
AH: none.
PA is an NIHR senior investigator, who is funded by the NIHR Oxford Health Biomedical Research Centre, the NIHR Oxford Biomedical Research Centre and the Oxford and Thames Valley ARC. He also works as a GP in the NHS and prescribes pharmacotherapy to patients. PA has published opinion/guidance pieces on e‐cigarettes for smoking cessation and on e‐cigarettes (
SCF is employed by the University of Leicester and is funded through grants for the NIHR Complex Reviews Support Unit (project number 14/178/29), NIHR Evidence Synthesis Group at CRSU and an NIHR Advanced Fellowship (PDF‐2018‐11‐ST2‐007) and is supported by the NIHR Applied Research Collaboration (ARC) East Midlands and Leicester NIHR Biomedical Research Centre (BRC). All funds are paid to the University of Leicester; however, SCF benefits in the form of a salary.
SZ: none.
SA is a Consultant in Respiratory and Intensive Care Medicine at University Hospitals of Leicester and National Specialist Advisor for Tobacco Dependency at NHS England. Neither SA nor these organisations have declared opinions or positions on this topic.
JHB is an editor for the Cochrane Tobacco Addiction Group; however, she was not involved in the editorial process for this review. JHB has written pieces for The Conversation and been interviewed by the media on the findings of Cochrane Reviews assessing the effects of treatments for smoking cessation. These outputs were evidence‐based and not based on personal opinion. JHB receives funding for two research grants on the topic of e‐cigarettes from Cancer Research UK, who have interests in people stopping smoking and run educational campaigns on the topic; paid to University of Oxford.
Figures




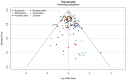
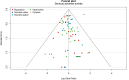
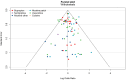
Update of
References
Additional references
ASH 2019
-
- ASH. Health inequalities and smoking. ash.org.uk/wp-content/uploads/2019/09/ASH-Briefing_Health-Inequalities.pdf (accessed 19 August 2021).
ASH 2021
-
- ASH. Use of e-cigarettes (vapes) among adults in Great Britain. ash.org.uk/wp-content/uploads/2021/06/Use-of-e-cigarettes-vapes-among-ad... (accessed 19 August 2021).
Balmford 2010
Cahill 2013
Cahill 2016
Chaiton 2016
Courtney 2021
Covidence [Computer program]
-
- Covidence. Version accessed 19 August 2021. Melbourne, Australia: Veritas Health Innovation. Available at covidence.org.
de Dios 2012
Ebbert 2010
Freeman 2018
-
- Freeman SC, Scott NW, Powell R, Johnston M, Sutton AJ, Cooper NJ. Component network meta-analysis identifies the most effective components of psychological preparation for adults undergoing surgery under general anesthesia. Journal of Clinical Epidemiology 2018;98:105-16. [DOI: 10.1016/j.jclinepi.2018.02.012] - DOI - PubMed
Hajizadeh 2023
Hartmann‐Boyce 2018
Hartmann‐Boyce 2019
Hartmann‐Boyce 2021a
Hartmann‐Boyce 2021b
-
- Hartmann-Boyce J, Livingstone-Banks J, Ordóñez-Mena JM, Fanshawe TR, Lindson N, Freeman SC, et al. Behavioural interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database of Systematic Reviews 2021, Issue 1. Art. No: CD013229. [DOI: 10.1002/14651858.CD013229.pub2] - DOI - PMC - PubMed
Hartmann‐Boyce 2022a
-
- Hartmann-Boyce J, Ordóñez-Mena JM, Livingstone‐Banks J, Fanshawe TR, Lindson N, Freeman SC, et al. Behavioural programmes for cigarette smoking cessation: investigating interactions between behavioural, motivational, and delivery components in a systematic review and component network meta-analysis. Addiction 2022;Jan:1-24. [DOI: 10.1111/add.15791] - DOI - PubMed
Hartmann‐Boyce 2022b
Hartmann‐Boyce 2023
Higgins 2011
-
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration. Available from training.cochrane.org/handbook/archive/v5.1/ 2011.
Higgins 2022
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Hoogendoorn 2010
Howes 2020
Lindson 2019
-
- Lindson N, Chepkin SC, Ye W, Fanshawe TR, Bullen C, Hartmann-Boyce J. Different doses, durations and modes of delivery of nicotine replacement therapy for smoking cessation. Cochrane Database of Systematic Reviews 2019, Issue 4. Art. No: CD013308. [DOI: 10.1002/14651858.CD013308] - DOI - PMC - PubMed
Livingstone‐Banks 2023
McNeill 2022
-
- McNeill A, Simonavičius E, Brose LS, Taylor E, East K, Zuikova E, et al. Nicotine vaping in England: an evidence update including health risks and perceptions. A report commissioned by the Office for Health Improvement and Disparities. London: Office for Health Improvement and Disparities. assets.publishing.service.gov.uk/government/uploads/system/uploads/attac... (accessed 20 December 2022).
Moore 2011
NCT03709823
-
- NCT03709823. Trial of cytisine in adult smokers [A multicenter, double-blind, randomized, placebo-controlled phase 2b trial of cytisine in adult smokers]. clinicaltrials.gov/ct2/show/NCT03709823 (first received 17 October 2018).
NICE 2021
-
- National Institute for Health and Care Excellence (NICE). Tobacco: preventing uptake, promoting quitting and treating dependence. NICE guideline [NG209]; 30 November 2021. Available at www.nice.org.uk/guidance/ng209 2021.
Nides 2021
Page 2021
Pesola 2002
Pirie 2013
Puhan 2014
R [Computer program]
-
- R: a language and environment for statistical computing. Version 4.1.1. Vienna, Austria: R Foundation for Statistical Computing, 2021. Available at www.R-project.org.
Raupach 2014
RCP 2018
-
- Royal College of Physicians (RCP). Hiding in Plain Sight: Treating Tobacco Dependency in the NHS. London: RCP, 2018.
Riley 2011
Sturtz 2005
-
- Sturtz S, Ligges U, Gelman AE. R2WinBUGS: a package for running WinBUGS from R. Journal of Statistical Software 2005;12(3):1-16. [DOI: 10.18637/jss.v012.i03] - DOI
Theodoulou 2023
-
- Theodoulou A, Chepkin SC, Ye W, Fanshawe TR, Bullen C, Hartmann-Boyce J, et al. Different doses, durations and modes of delivery of nicotine replacement therapy for smoking cessation. Cochrane Database of Systematic Reviews 2023, Issue 6. Art. No: CD013308. [DOI: 10.1002/14651858.CD013308.pub2] - DOI - PMC - PubMed
Thomas 2021
-
- Thomas KH, Dalili MN, López-López JA, Keeney E, Phillippo DM, Munafò MR, et al. Comparative clinical effectiveness and safety of tobacco cessation pharmacotherapies and electronic cigarettes: a systematic review and network meta-analysis of randomized controlled trials. Addiction 2022;117(4):861-76. [DOI: 10.1111/add.15675] - DOI - PMC - PubMed
Warner 2023
West 2005
WHO 2019
-
- World Health Organization. WHO report on the global tobacco epidemic 2019: offer help to quit tobacco use. www.who.int/publications/i/item/9789241516204 (accessed 19 August 2021).
WHO 2021a
-
- World Health Organization. Tobacco. www.who.int/news-room/fact-sheets/detail/tobacco (accessed 19 August 2021).
WHO 2021b
-
- World Health Organization Expert Committee. The selection and use of essential medicines: report of the WHO Expert Committee on Selection and Use of Essential Medicines, 2021 (including the 22nd WHO Model List of Essential Medicines and the 8th WHO Model List of Essential Medicines for Children). Geneva: World Health Organization; 2021. Report No.: 1035 2021.
WinBUGS [Computer program]
-
- WinBUGS. Version 1.4.3. Cambridge, UK: MRC Biostatistics Unit, 2007. Available at: www.mrc-bsu.cam.ac.uk/software/bugs/the-bugs-project-winbugs/.
References to other published versions of this review
Lindson 2022
-
- Lindson N, Theodoulou A, Livingstone-Banks J, Aveyard P, Fanshawe TR, Ordóñez-Mena JM, et al. Pharmacological and electronic cigarette interventions for smoking cessation in adults: component network meta-analyses. Cochrane Database of Systematic Reviews 2022, Issue 3. Art. No: CD015226. [DOI: 10.1002/14651858.CD015226] - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

