Effects of sotatercept on haemodynamics and right heart function: analysis of the STELLAR trial
- PMID: 37696565
- PMCID: PMC10512088
- DOI: 10.1183/13993003.01107-2023
Effects of sotatercept on haemodynamics and right heart function: analysis of the STELLAR trial
Abstract
Background: In the phase 3 STELLAR trial, sotatercept, an investigational first-in-class activin signalling inhibitor, demonstrated beneficial effects on 6-min walk distance and additional efficacy endpoints in pre-treated participants with pulmonary arterial hypertension (PAH).
Methods: This post hoc analysis evaluated data from right heart catheterisation (RHC) and echocardiography (ECHO) obtained from the STELLAR trial. Changes from baseline in RHC and ECHO parameters were assessed at 24 weeks. An analysis of covariance (ANCOVA) model was used to estimate differences in least squares means with treatment and randomisation stratification (mono/double versus triple therapy; World Health Organization functional class II versus III) as fixed factors, and baseline value as covariate.
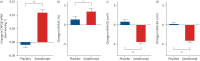
Results: Relative to placebo, treatment with sotatercept led to significant (all p<0.0001 except where noted) improvements from baseline in mean pulmonary artery (PA) pressure (-13.9 mmHg), pulmonary vascular resistance (-254.8 dyn·s·cm-5), mean right atrial pressure (-2.7 mmHg), mixed venous oxygen saturation (3.84%), PA elastance (-0.42 mmHg·mL-1·beat-1), PA compliance (0.58 mL·mmHg-1), cardiac efficiency (0.48 mL·beat-1·mmHg-1), right ventricular (RV) work (-0.85 g·m) and RV power (-32.70 mmHg·L·min-1). ECHO showed improvements in tricuspid annular plane systolic excursion (TAPSE) to systolic pulmonary artery pressure ratio (0.12 mm·mmHg-1), end-systolic and end-diastolic RV areas (-4.39 cm2 and -5.31 cm2, respectively), tricuspid regurgitation and RV fractional area change (2.04% p<0.050). No significant between-group changes from baseline were seen for TAPSE, heart rate, cardiac output, stroke volume or their indices.
Conclusion: In pre-treated patients with PAH, sotatercept demonstrated substantial improvements in PA pressures, PA compliance, PA-RV coupling and right heart function.
Trial registration: ClinicalTrials.gov NCT04576988.
Copyright ©The authors 2023.
Conflict of interest statement
Conflict of interest: R. Souza has served as consultant for Acceleron Pharma, Inc., Bayer Healthcare Pharmaceuticals Inc. and Janssen Biotech, Inc. (fees paid to self). D.B. Badesch has received grants/contracts from Acceleron Pharma, Inc., Merck & Co., Inc. (Rahway, NJ, USA), Altavant and United Therapeutics (all paid to institution, clinical trial), received payment as consultant from Acceleron Pharma, Inc., Merck & Co., Inc. (Rahway, NJ, USA) and Aerovate Inc. (paid through institution), and the author's spouse or partner has stock in Johnson & Johnson Health Care Systems Inc. H.A. Ghofrani has served as consultant for Aerovate, Altavant, Bayer Healthcare, Gossamer Bio, Janssen Diagnostics, LLC, Merck & Co., Inc. (Rahway, NJ, USA) and Pfizer (fees paid to self), and is an employee of Justus Liebig University Giessen, Germany. J.S.R. Gibbs has served a consultant for Acceleron Pharma and Merck & Co., Inc. (Rahway, NJ, USA) (fees paid to self), served in data and safety monitoring boards for Actelion Pharmaceuticals, Fundação Bial, GossamerBio and Merck & Co., Inc. (Rahway, NJ, USA) (fees paid to self), served in end point review committee for Actelion Pharmaceuticals, Aerovate, Janssen Biotech, Pfizer Pharma GMBH and United Therapeutics Corporation (fees paid to self), and served as Chair of ERN-Lung Functional Committee Patient Reported Outcomes for ERN Lung. M. Gomberg-Maitland has served as a consultant for Acceleron Pharma and Merck & Co., Inc. (Rahway, NJ, USA), Aerami, Bayer HealthCare Pharmaceuticals Inc., Janssen Biotech, Keros and United Therapeutics Corporation (fees paid to self), has received grant/contract from Aerovate, Altavant, Acceleron Pharma and Merck & Co., Inc. (fees paid to institution), and the author's spouse is an employee of Intellia Therapeutics. V.V. McLaughlin has served as a consultant for Aerami, Aerovate, Altavant, Bayer Healthcare, Caremark, Corvista, Gossamer Bio, Janssen Biotech, Merck & Co., Inc. (Rahway, NJ, USA) and United Therapeutics (fees paid to self), received grants/contracts from Aerovate, Altavant, Gossamer Bio, Janssen Biotech, Merck & Co., Inc. (Rahway, NJ, USA) and Sonovie (paid to institution), served as fiduciary officer, board of directors, for Clene (fees paid to self), and is an employee of the University of Michigan. I.R. Preston has served as a steering committee member for Acceleron Pharma, Liquidia and United Therapeutics (fees paid to self), served as scientific advisory board member for Aerovate, Altavant and Gossamer (fees paid to self), served as consultant for Janssen Global Services, LLC and Respira Therapeutics (fees paid to self), and served as principal investigator for Janssen Global Services, LLC and United Therapeutics (fees paid to self). A.B. Waxman has served as consultant for ARIA-CV, Goassamer, Merck & Co., Inc. (Rahway, NJ, USA) and United Therapeutics Corporation (fees paid to self), received grants/contracts from AI Therapeutics, Inc. (fees paid to self), and served on a data and safety monitoring committee for Insmed, Inc. (fees paid to self). E. Grünig has served as consultant for Actelion Pharmaceuticals (fees paid to self), and served as speaker and/or consultant for Bayer Healthcare, Ferrer, GEBRO, GlaxoSmithKline, Janssen Biotech, Merck Sharp & Dohme (MSD) and OMT (fees paid to self). G. Kopeć has served as consultant for Acceleron Pharma, Inc. and Janssen Global Services, LLC, on a scientific advisory board (fees paid to self), served as PI in a clinical study for Acceleron Pharma, Inc., Bayer and Janssen Global Services, LLC (fees paid to self), served as a speaker for Bayer, Janssen Global Services, LLC and Merck & Co., Inc. (Rahway, NJ, USA) (fees paid to self), served as investigator for Janssen Global Services, LLC (fees paid to self), and received travel fees from Janssen Global Services, LLC and Merck & Co., Inc. (Rahway, NJ, USA) (paid to self). G. Meyer has served as consultant for Bayer Healthcare and Janssen Biotech (fees paid to self), and received grants/contracts from Bayer Healthcare (paid to institution). K.M. Olsson has served as a consultant for Acceleron Pharma, Inc., Actelion Pharmaceuticals, AOP Orphan, Bayer, Ferrer Pharma, Janssen Global Services, LLC and Merck & Co., Inc. (Rahway, NJ, USA) (fees paid to self). S. Rosenkranz has served as consultant for Abbott Fund, Acceleron Pharma, Inc., Actelion Pharmaceuticals, Aerovate, Altavant, AOP Orphan, AstraZeneca, Bayer, Boehringer Ingelheim, Edwards Lifesciences, Ferrer, Gossamer, Janssen, MSD and United Therapeutics (fees paid to self), and received grants/contracts from Actelion Pharmaceuticals, AstraZeneca, Bayer and Janssen (paid to institution). J. Lin, A.O. Johnson-Levonas and J. de Oliveira Pena are employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and own stock/stock shares in Merck & Co., Inc., Rahway, NJ, USA. M. Humbert has served as consultant for Acceleron Pharma, Inc., Aerovate, Altavant, AOP Orphan, Bayer, Chiesi Farmaceutici, Ferrer, Janssen Pharmaceuticals, Merck & Co., Inc. (Rahway, NJ, USA), MorphogenIX and United Therapeutics Corporation (fees paid to self). M.M. Hoeper has served as consultant for Acceleron Pharma, Inc., Actelion Pharmaceuticals, AOP Orphan, Bayer Healthcare, Ferrer, GossamerBio, Janssen Global Services, LLC and Merck & Co., Inc. (Rahway, NJ, USA) (fees paid to self).
Figures





Comment in
-
Reducing the pressure in pulmonary arterial hypertension: sotatercept, haemodynamics and the right ventricle.Eur Respir J. 2023 Sep 21;62(3):2301513. doi: 10.1183/13993003.01513-2023. Print 2023 Sep. Eur Respir J. 2023. PMID: 37696566 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
