Deficiency of endothelial sirtuin1 in mice stimulates skeletal muscle insulin sensitivity by modifying the secretome
- PMID: 37696839
- PMCID: PMC10495425
- DOI: 10.1038/s41467-023-41351-1
Deficiency of endothelial sirtuin1 in mice stimulates skeletal muscle insulin sensitivity by modifying the secretome
Abstract
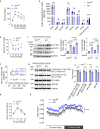
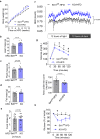
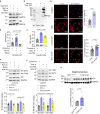
Downregulation of endothelial Sirtuin1 (Sirt1) in insulin resistant states contributes to vascular dysfunction. Furthermore, Sirt1 deficiency in skeletal myocytes promotes insulin resistance. Here, we show that deletion of endothelial Sirt1, while impairing endothelial function, paradoxically improves skeletal muscle insulin sensitivity. Compared to wild-type mice, male mice lacking endothelial Sirt1 (E-Sirt1-KO) preferentially utilize glucose over fat, and have higher insulin sensitivity, glucose uptake, and Akt signaling in fast-twitch skeletal muscle. Enhanced insulin sensitivity of E-Sirt1-KO mice is transferrable to wild-type mice via the systemic circulation. Endothelial Sirt1 deficiency, by inhibiting autophagy and activating nuclear factor-kappa B signaling, augments expression and secretion of thymosin beta-4 (Tβ4) that promotes insulin signaling in skeletal myotubes. Thus, unlike in skeletal myocytes, Sirt1 deficiency in the endothelium promotes glucose homeostasis by stimulating skeletal muscle insulin sensitivity through a blood-borne mechanism, and augmented secretion of Tβ4 by Sirt1-deficient endothelial cells boosts insulin signaling in skeletal muscle cells.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

