Synthetic circuits based on split Cas9 to detect cellular events
- PMID: 37696879
- PMCID: PMC10495424
- DOI: 10.1038/s41598-023-41367-z
Synthetic circuits based on split Cas9 to detect cellular events
Abstract
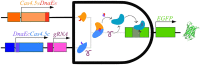
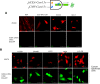
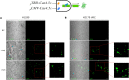
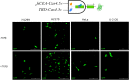
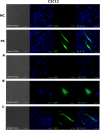
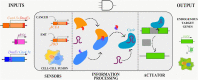
Synthetic biology involves the engineering of logic circuit gates that process different inputs to produce specific outputs, enabling the creation or control of biological functions. While CRISPR has become the tool of choice in molecular biology due to its RNA-guided targetability to other nucleic acids, it has not been frequently applied to logic gates beyond those controlling the guide RNA (gRNA). In this study, we present an adaptation of split Cas9 to generate logic gates capable of sensing biological events, leveraging a Cas9 reporter (EGxxFP) to detect occurrences such as cancer cell origin, epithelial to mesenchymal transition (EMT), and cell-cell fusion. First, we positioned the complementing halves of split Cas9 under different promoters-one specific to cancer cells of epithelial origin (phCEA) and the other a universal promoter. The use of self-assembling inteins facilitated the reconstitution of the Cas9 halves. Consequently, only cancer cells with an epithelial origin activated the reporter, exhibiting green fluorescence. Subsequently, we explored whether this system could detect biological processes such as epithelial to mesenchymal transition (EMT). To achieve this, we designed a logic gate where one half of Cas9 is expressed under the phCEA, while the other is activated by TWIST1. The results showed that cells undergoing EMT effectively activated the reporter. Next, we combined the two inputs (epithelial origin and EMT) to create a new logic gate, where only cancer epithelial cells undergoing EMT activated the reporter. Lastly, we applied the split-Cas9 logic gate as a sensor of cell-cell fusion, both in induced and naturally occurring scenarios. Each cell type expressed one half of split Cas9, and the induction of fusion resulted in the appearance of multinucleated syncytia and the fluorescent reporter. The simplicity of the split Cas9 system presented here allows for its integration into various cellular processes, not only as a sensor but also as an actuator.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

