An inspired microenvironment of cell replicas to induce stem cells into keratocyte-like dendritic cells for corneal regeneration
- PMID: 37696883
- PMCID: PMC10495344
- DOI: 10.1038/s41598-023-42359-9
An inspired microenvironment of cell replicas to induce stem cells into keratocyte-like dendritic cells for corneal regeneration
Abstract
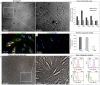
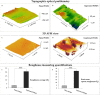
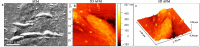
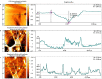
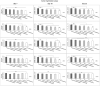
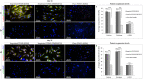
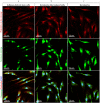
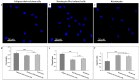
Corneal stromal disorders due to the loss of keratocytes can affect visual impairment and blindness. Corneal cell therapy is a promising therapeutic strategy for healing corneal tissue or even enhancing corneal function upon advanced disorders, however, the sources of corneal keratocytes are limited for clinical applications. Here, the capacity of cell-imprinted substrates fabricated by molding human keratocyte templates to induce differentiation of human adipose-derived stem cells (hADSCs) into keratocytes, is presented. Keratocytes are isolated from human corneal stroma and grown to transmit their ECM architecture and cell-like topographies to a PDMS substrate. The hADSCs are then seeded on cell-imprinted substrates and their differentiation to keratocytes in DMEM/F12 (with and without chemical factors) are evaluated by real-time PCR and immunocytochemistry. The mesenchymal stem cells grown on patterned substrates present gene and protein expression profiles similar to corneal keratocytes. In contrast, a negligible expression of myofibroblast marker in the hADSCs cultivated on the imprinted substrates, is observed. Microscopic analysis reveals dendritic morphology and ellipsoid nuclei similar to primary keratocytes. Overall, it is demonstrated that biomimetic imprinted substrates would be a sufficient driver to solely direct the stem cell fate toward target cells which is a significant achievement toward corneal regeneration.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Combination of natural scaffolds and conditional medium to induce the differentiation of adipose-derived mesenchymal stem cells into keratocyte-like cells and its safety evaluation in the animal cornea.Tissue Cell. 2023 Jun;82:102117. doi: 10.1016/j.tice.2023.102117. Epub 2023 May 20. Tissue Cell. 2023. PMID: 37267821
-
Keratocyte biology.Exp Eye Res. 2020 Jul;196:108062. doi: 10.1016/j.exer.2020.108062. Epub 2020 May 19. Exp Eye Res. 2020. PMID: 32442558 Review.
-
Derivation of Corneal Keratocyte-Like Cells from Human Induced Pluripotent Stem Cells.PLoS One. 2016 Oct 28;11(10):e0165464. doi: 10.1371/journal.pone.0165464. eCollection 2016. PLoS One. 2016. PMID: 27792791 Free PMC article.
-
Engineered substrates with imprinted cell-like topographies induce direct differentiation of adipose-derived mesenchymal stem cells into Schwann cells.Artif Cells Nanomed Biotechnol. 2019 Dec;47(1):1022-1035. doi: 10.1080/21691401.2019.1586718. Artif Cells Nanomed Biotechnol. 2019. PMID: 30942113
-
Concise review: Stem cells in the corneal stroma.Stem Cells. 2012 Jun;30(6):1059-63. doi: 10.1002/stem.1100. Stem Cells. 2012. PMID: 22489057 Free PMC article. Review.
Cited by
-
Analyzing the Antihyperglycemic Effect of Cissus quadrangularis and Bacopa monnieri on 3T3-L1 Cell Lines.Cureus. 2024 Jan 21;16(1):e52661. doi: 10.7759/cureus.52661. eCollection 2024 Jan. Cureus. 2024. PMID: 38380214 Free PMC article.
-
Drug delivery strategies to improve the treatment of corneal disorders.Heliyon. 2025 Jan 10;11(2):e41881. doi: 10.1016/j.heliyon.2025.e41881. eCollection 2025 Jan 30. Heliyon. 2025. PMID: 39897787 Free PMC article. Review.
-
Directed Differentiation of Adipose-Derived Stem Cells Using Imprinted Cell-Like Topographies as a Growth Factor-Free Approach.Stem Cell Rev Rep. 2024 Oct;20(7):1752-1781. doi: 10.1007/s12015-024-10767-7. Epub 2024 Jul 27. Stem Cell Rev Rep. 2024. PMID: 39066936 Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

