Impact of a TLR9 agonist and broadly neutralizing antibodies on HIV-1 persistence: the randomized phase 2a TITAN trial
- PMID: 37696935
- PMCID: PMC10579101
- DOI: 10.1038/s41591-023-02547-6
Impact of a TLR9 agonist and broadly neutralizing antibodies on HIV-1 persistence: the randomized phase 2a TITAN trial
Abstract
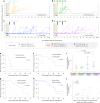
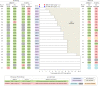
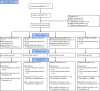
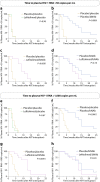
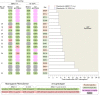
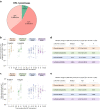
Inducing antiretroviral therapy (ART)-free virological control is a critical step toward a human immunodeficiency virus type 1 (HIV-1) cure. In this phase 2a, placebo-controlled, double-blinded trial, 43 people (85% males) with HIV-1 on ART were randomized to (1) placebo/placebo, (2) lefitolimod (TLR9 agonist)/placebo, (3) placebo/broadly neutralizing anti-HIV-1 antibodies (bNAbs) or (4) lefitolimod/bNAb. ART interruption (ATI) started at week 3. Lefitolimod was administered once weekly for the first 8 weeks, and bNAbs were administered twice, 1 d before and 3 weeks after ATI. The primary endpoint was time to loss of virologic control after ATI. The median delay in time to loss of virologic control compared to the placebo/placebo group was 0.5 weeks (P = 0.49), 12.5 weeks (P = 0.003) and 9.5 weeks (P = 0.004) in the lefitolimod/placebo, placebo/bNAb and lefitolimod/bNAb groups, respectively. Among secondary endpoints, viral doubling time was slower for bNAb groups compared to non-bNAb groups, and the interventions were overall safe. We observed no added benefit of lefitolimod. Despite subtherapeutic plasma bNAb levels, 36% (4/11) in the placebo/bNAb group compared to 0% (0/10) in the placebo/placebo group maintained virologic control after the 25-week ATI. Although immunotherapy with lefitolimod did not lead to ART-free HIV-1 control, bNAbs may be important components in future HIV-1 curative strategies. ClinicalTrials.gov identifier: NCT03837756 .
© 2023. The Author(s).
Conflict of interest statement
M.C.N. is listed as an inventor on patents for the antibodies 3BNC117 and 10-1074. All other authors declare no competing interests.
Figures






References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

