Effectiveness and safety of fluocinolone acetonide intravitreal implant in diabetic macular edema patients considered insufficiently responsive to available therapies (REACT): a prospective, non-randomized, and multicenter study
- PMID: 37697082
- PMCID: PMC10724319
- DOI: 10.1007/s10792-023-02864-2
Effectiveness and safety of fluocinolone acetonide intravitreal implant in diabetic macular edema patients considered insufficiently responsive to available therapies (REACT): a prospective, non-randomized, and multicenter study
Abstract
Objective: To assess the effectiveness and safety of the intravitreal fluocinolone-acetonide implant (FAc-i) in patients with chronic diabetic macular edema who did not sufficiently respond to other available therapies.
Methods: This was a multicenter, prospective, non-randomized, and phase-IV observational study conducted on patients with recurrent-DME who were insufficient responders to currently available therapies (REACT-Study). The primary end-point was the mean change in best-corrected-visual-acuity from baseline to month-24 values.
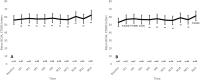
Results: Thirty-one eyes from 31 patients were included in the study. Mean age was 68.0 ± 7.7 years, and 10 (32.3%) were women. Study patients had received 5.3 ± 7.3 previous DME treatments before starting the study. In the overall study sample, BCVA improved from 56.1 ± 12.3 letters at baseline to 62.4 ± 17.0 letters at month-24 (p = 0.0510). The eyes with a baseline BCVA < 70 ETDRS letters had a significant improvement in BCVA from 53.2 ± 10.2 letters at baseline to 61.5 ± 17.9 letters at month-24 (p = 0.0165). In the overall study population, central-subfoveal-thickness (CST) was significantly reduced from 474.0 ± 135.1 µm at baseline to 333.4 ± 135.6 at month-24 (p < 0.0001). Similarly, macular-volume (MV) was significantly reduced from 10.7 ± 2.7 mm3 at baseline to 9.6 ± 2.9 mm3 (p = 0.0027) at month-24. Among the 31 study eyes, 19 (61.3%) required an additional treatment for DME. Throughout the study, 9 (29.0%) eyes required ocular hypotensive medication for controlling their intraocular-pressure and 5 (16.1%) eyes underwent cataract surgery.
Conclusions: In DME eyes who did not sufficiently respond to previous therapies, the FAc-i was associated with an improvement in visual and anatomic outcomes. There were no unexpected adverse-events.
Trial registration number: EudraCT identifier: 2016-001680-37.
Keywords: Corticoids; Diabetic macular edema; Fluocinolone acetonide intravitreal implant; VEGF inhibitors.
© 2023. The Author(s).
Conflict of interest statement
Dr Ruiz-Moreno has received a Grant from Alimera during the conduct of the study. Neither honoraria nor payments were made for authorship of this article. The other authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Figures


References
-
- Alessio G, Boscia F, Caporossi A, Panozzo G, Reibaldi M, Staurenghi G, Varano M, Bandello F. Dexamethasone implants in patients with diabetic macular edema undergoing cataract surgery: Italian expert panel consensus statements. Eur J Ophthalmol. 2021;31(3):1122–1127. doi: 10.1177/1120672120939500. - DOI - PubMed
-
- Campochiaro PA, Nguyen QD, Hafiz G, Bloom S, Brown DM, Busquets M, Ciulla T, Feiner L, Sabates N, Billman K, Kapik B, Green K, Kane FE, FAMOUS Study Group Aqueous levels of fluocinolone acetonide after administration of fluocinolone acetonide inserts or fluocinolone acetonide implants. Ophthalmology. 2013;120(3):583–587. doi: 10.1016/j.ophtha.2012.09.014. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

