Tapinarof Cream 1% Once Daily for the Treatment of Plaque Psoriasis: Case Photography of Clinical Outcomes from Three Phase 3 Trials
- PMID: 37697121
- PMCID: PMC10539260
- DOI: 10.1007/s13555-023-01008-9
Tapinarof Cream 1% Once Daily for the Treatment of Plaque Psoriasis: Case Photography of Clinical Outcomes from Three Phase 3 Trials
Abstract
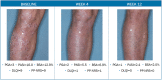
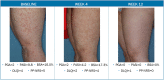
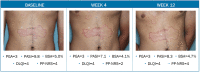
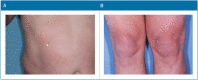
Tapinarof cream 1% (VTAMA®; Dermavant Sciences, Inc.) is a non-steroidal, topical, aryl hydrocarbon receptor agonist approved by the US Food and Drug Administration (FDA) to treat plaque psoriasis in adults and under investigation for the treatment of psoriasis in children down to 2 years of age, and for atopic dermatitis in adults and children down to 2 years of age. The PSOARING phase 3 clinical trial program evaluated tapinarof cream 1% once daily (QD) in adults with mild to severe plaque psoriasis for up to 52 weeks (NCT03956355, NCT03983980, NCT04053387). Here we present case photography documenting outcomes in the PSOARING trials. Cases illustrate various outcomes across different body areas, including responses meeting the formal FDA-mandated regulatory endpoint of a Physician Global Assessment (PGA) score of 0 (clear) or 1 (almost clear) and a decrease of at least 2 points from baseline at week 12, meaningful clinical improvement not meeting this formal endpoint, patient-reported outcomes, and pre-specified adverse events of special interest (AESIs). Tapinarof cream 1% QD demonstrated rapid and highly statistically significant efficacy, with improvements in disease activity and quality of life. In addition, a high rate (40.9%; n = 312/763) of complete disease clearance (PGA = 0) was achieved, and improvements exceeding National Psoriasis Foundation treatment goals were demonstrated. After first achieving complete disease clearance (PGA = 0), patients treated with tapinarof experienced an approximately 4-month remittive effect off therapy. Incidence and severity of folliculitis and contact dermatitis AESIs were generally mild or moderate, localized to the site of application, and associated with low discontinuation rates. Medical images are of importance in trials of dermatologic therapies to inform clinical decision-making and enhance patient assessment. Tapinarof cream 1% QD is efficacious and well tolerated in patients with mild to severe plaque psoriasis, with clinically relevant improvements seen early in the course of treatment.Clinicaltrials.gov numbers: NCT03956355, NCT03983980, NCT04053387.
Keywords: Aryl hydrocarbon receptor agonist; Case photography; PSOARING; Phase 3 randomized controlled trials; Plaque psoriasis; Tapinarof cream 1% once daily; Topical therapy.
© 2023. The Author(s).
Conflict of interest statement
Seemal R. Desai has served as a consultant and investigator for Dermavant Sciences, Inc.; he also serves in multiple other leadership and industry roles unrelated to tapinarof cream 1%. Linda Stein Gold has served as a consultant, and/or has received payment for the development of educational presentations, and/or has received grants from Amgen, Arcutis, Bristol Myers Squibb, Dermavant Sciences, Inc., Eli Lilly, LEO Pharma, Ortho Dermatologics, Pfizer, and UCB Biopharma. Michael C. Cameron has served as a consultant, advisor, or speaker for AbbVie, Bristol Myers Squibb, Dermavant Sciences, Inc., Eli Lilly, EPI Health, Evelo Biosciences, Incyte, Journey Medical, LEO Pharma, Regeneron, and Ortho Pharmaceuticals. Alexandra Golant has received consulting or speaking fees from AbbVie, Amgen, Arcutis, Dermavant Sciences, Inc., Eli Lilly, Evelo Biosciences, Incyte, Janssen, LEO Pharma, Regeneron, and Sanofi. G. Michael Lewitt has served as a consultant, speaker, investigator, or advisory board member and/or has received grants from AbbVie, Amgen, Inc., Bristol Myers Squibb, Dermavant Sciences, Inc., DermTech, Eli Lilly, Galderma, LEO Pharma, Janssen, Novan, Inc., Pfizer, Ortho Dermatologics, and UCB Biopharma. Matthew J. Bruno has served as a consultant and/or received payment for promotional presentations from AbbVie, Almirall, Bristol Myers Squibb, Dermavant Sciences, Inc., EPI Health, Journey Medical Corporation, Mayne Pharma, Medimetriks Pharmaceuticals, Pfizer, Regeneron/Sanofi-Genzyme, and Sun Pharmaceuticals. George Martin has served as a speaker and/or consultant and/or has been involved in scientific advisory boards for AbbVie, Almirall, Alumis, Bristol Myers Squibb, Celgene, Dermavant Sciences, Inc., DUSA/Sun, Eli Lilly, Evelo, Galderma, Horizon, Incyte, Janssen, LEO Pharma, Nobelpharma, Ortho/Bausch Health, Organogenesis, Pfizer, Trevi, and UCB Biopharma. Philip M. Brown, David S. Rubenstein, Victoria Butners, and Anna M. Tallman are employees of Dermavant Sciences, Inc., with stock options.
Figures







References
-
- Stein GL. Topical therapies for psoriasis: improving management strategies and patient adherence. Semin Cutan Med Surg. 2016;35(2 Suppl 2):S36–S44. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

