Transcriptional and epigenetic decoding of the microglial aging process
- PMID: 37697166
- PMCID: PMC10570141
- DOI: 10.1038/s43587-023-00479-x
Transcriptional and epigenetic decoding of the microglial aging process
Erratum in
-
Publisher Correction: Transcriptional and epigenetic decoding of the microglial aging process.Nat Aging. 2024 Feb;4(2):276. doi: 10.1038/s43587-024-00571-w. Nat Aging. 2024. PMID: 38200274 Free PMC article. No abstract available.
Abstract
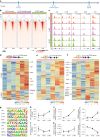
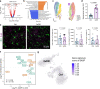
As important immune cells, microglia undergo a series of alterations during aging that increase the susceptibility to brain dysfunctions. However, the longitudinal characteristics of microglia remain poorly understood. In this study, we mapped the transcriptional and epigenetic profiles of microglia from 3- to 24-month-old mice. We first discovered unexpected sex differences and identified age-dependent microglia (ADEM) genes during the aging process. We then compared the features of aging and reactivity in female microglia at single-cell resolution and epigenetic level. To dissect functions of aged microglia excluding the influence from other aged brain cells, we established an accelerated microglial turnover model without directly affecting other brain cells. By this model, we achieved aged-like microglia in non-aged brains and confirmed that aged-like microglia per se contribute to cognitive decline. Collectively, our work provides a comprehensive resource for decoding the aging process of microglia, shedding light on how microglia maintain brain functions.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
















Comment in
-
Dissecting microglial aging and creating a model of aged microglia in a non-aged brain.Nat Aging. 2023 Oct;3(10):1185-1186. doi: 10.1038/s43587-023-00487-x. Nat Aging. 2023. PMID: 37697167 No abstract available.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

