Differences between CEUS LI-RADS and CECT LI-RADS in the diagnosis of focal liver lesions in patients at risk for HCC
- PMID: 37697248
- PMCID: PMC10496202
- DOI: 10.1186/s12880-023-01088-1
Differences between CEUS LI-RADS and CECT LI-RADS in the diagnosis of focal liver lesions in patients at risk for HCC
Abstract
Objectives: To compare the inter-modality consistency and diagnostic performances of the contrast-enhanced ultrasound (CEUS) Liver Imaging Reporting and Data System (LI-RADS) and contrast-enhanced computed tomography (CECT) LI-RADS in patients at risk for hepatocellular carcinoma (HCC), so as to help clinicians to select a more appropriate modality to follow the focal liver lesions (FLLs).
Methods: This retrospective study included untreated 277 FLLs from 247 patients who underwent both CEUS and CECT within 1 month. The ultrasound contrast medium used was SonoVue. FLL categories were independently assigned by two ultrasound physicians and two radiologists using CEUS LI-RADS v2017 and CECT LI-RADS v2018, respectively. The diagnostic performances of CEUS and CECT LI-RADS were evaluated using sensitivity, specificity, positive predictive value (PPV), and negative predictive value. Cohen's Kappa was employed to evaluate the concordance of the LI-RADS category.
Results: The inter-modality consistency for CEUS and CECT LI-RADS was 0.31 (p < 0.001). HCC was more frequently observed in CECT LR-3 and LR-4 hepatic lesions than in CEUS (7.3% vs. 19.5%, p < 0.001). The specificity and PPV of CEUS and CECT LR-5 for the diagnosis of HCC were 89.5%, 95.0%, and 82.5%, 94.4%, respectively. The sensitivity of CEUS LR-5 + LR-M for the diagnosis of hepatic malignancies was higher than that of CECT (93.7% vs. 82.7%, p < 0.001). The specificity and PPV of CEUS LR-M for the diagnosis of non-HCC malignancies were lower than those of CECT (59.7% vs. 95.5%, p < 0.001; 23.4% vs. 70.3%, p < 0.001).
Conclusions: The inter-modality consistency between the CEUS and CECT LI-RADS categories is fair. CEUS LI-RADS was more sensitive than CECT LI-RADS in terms of identifying hepatic malignancies, but weaker in terms of separating HCC from non-HCC malignancies.
Keywords: Computed tomography; Hepatocellular carcinoma; Liver Imaging Reporting and Data System; Ultrasonography.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests.
Figures

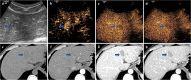
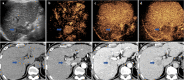
References
-
- Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, staging, and management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the study of Liver Diseases. Hepatology. 2018;68(2):723–50. doi: 10.1002/hep.29913. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

