RNF8 ubiquitylation of XRN2 facilitates R-loop resolution and restrains genomic instability in BRCA1 mutant cells
- PMID: 37697435
- PMCID: PMC10602868
- DOI: 10.1093/nar/gkad733
RNF8 ubiquitylation of XRN2 facilitates R-loop resolution and restrains genomic instability in BRCA1 mutant cells
Abstract
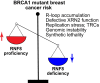
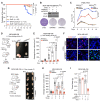
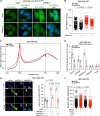
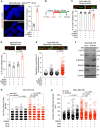
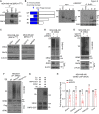
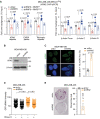
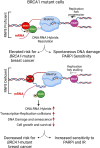
Breast cancer linked with BRCA1/2 mutations commonly recur and resist current therapies, including PARP inhibitors. Given the lack of effective targeted therapies for BRCA1-mutant cancers, we sought to identify novel targets to selectively kill these cancers. Here, we report that loss of RNF8 significantly protects Brca1-mutant mice against mammary tumorigenesis. RNF8 deficiency in human BRCA1-mutant breast cancer cells was found to promote R-loop accumulation and replication fork instability, leading to increased DNA damage, senescence, and synthetic lethality. Mechanistically, RNF8 interacts with XRN2, which is crucial for transcription termination and R-loop resolution. We report that RNF8 ubiquitylates XRN2 to facilitate its recruitment to R-loop-prone genomic loci and that RNF8 deficiency in BRCA1-mutant breast cancer cells decreases XRN2 occupancy at R-loop-prone sites, thereby promoting R-loop accumulation, transcription-replication collisions, excessive genomic instability, and cancer cell death. Collectively, our work identifies a synthetic lethal interaction between RNF8 and BRCA1, which is mediated by a pathological accumulation of R-loops.
© The Author(s) 2023. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures

References
-
- Ashworth A., Lord C.J.. Synthetic lethal therapies for cancer: what's next after PARP inhibitors?. Nat. Rev. Clin. Oncol. 2018; 15:564–576. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

