Therapeutic mechanism of Liangxue-Guyuan-Yishen decoction on intestinal stem cells and tight junction proteins in gastrointestinal acute radiation syndrome rats
- PMID: 37697698
- PMCID: PMC10665307
- DOI: 10.1093/jrr/rrad065
Therapeutic mechanism of Liangxue-Guyuan-Yishen decoction on intestinal stem cells and tight junction proteins in gastrointestinal acute radiation syndrome rats
Abstract
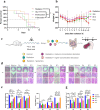
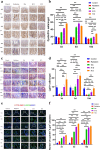
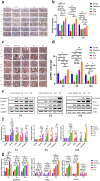
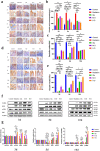
On the basis of the previous research, the Traditional Chinese Medicine theory was used to improve the drug composition for gastrointestinal acute radiation syndrome (GI-ARS). The purpose of this study was to study the therapeutic mechanism of Liangxue-Guyuan-Yishen decoction (LGYD) on GI-ARS and to provide a new scheme for the treatment of radiation injury. Here, we investigated the effects of LGYD on intestinal stem cells (ISCs) in a GI-ARS rat model. Rat health and survival and the protective efficacy of LGYD on the intestines were analyzed. The active principles in LGYD were detected using liquid chromatography-mass spectrometry (LC-MS). ISC proliferation, intestinal epithelial tight junction (TJ) protein expression and regulatory pathways were explored using immunohistochemistry, western blotting (WB) and reverse transcription quantitative polymerase chain reaction (RT-qPCR), respectively. Involvement of the WNT and MEK/ERK pathways in intestinal recovery was screened using network pharmacology analysis and validated by WB and RT-qPCR. LGYD administration significantly improved health and survival in GI-ARS rats. Pathological analysis showed that LGYD ameliorated radiation-induced intestinal injury and significantly promoted LGR5+ stem cell regeneration in the intestinal crypts, upregulated TJ protein, and accelerated crypt reconstruction in the irradiated rats. LC-MS revealed ≥13 constituents that might contribute to LGYD's protective effects. Collectively, LGYD can promote crypt cell proliferation and ISCs after radiation damage, the above effect may be related to WNT and MEK/ERK pathway.
Keywords: Chinese herbal medicine; gastrointestinal acute radiation syndrome; intestinal stem cell; tight junction protein.
© The Author(s) 2023. Published by Oxford University Press on behalf of The Japanese Radiation Research Society and Japanese Society for Radiation Oncology.
Conflict of interest statement
All authors disclosed no relevant relationships.
Figures

References
-
- Maisin JR. Bacq and Alexander award lecture chemical radioprotection: past, present and future prospects. Int J Radiat Biol 1998;73:443–50. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

