Semisynthesis of A6-A11 lactam insulin
- PMID: 37697741
- PMCID: PMC10909544
- DOI: 10.1002/psc.3542
Semisynthesis of A6-A11 lactam insulin
Abstract
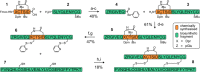
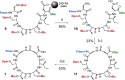
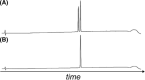
Insulin replacement therapy is essential for the management of diabetes. However, despite the relative success of this therapeutic strategy, there is still a need to improve glycaemic control and the overall quality of life of patients. This need has driven research into orally available, glucose-responsive and rapid-acting insulins. A key consideration during analogue development is formulation stability, which can be improved via the replacement of insulin's A6-A11 disulfide bond with stable mimetics. Unfortunately, analogues such as these require extensive chemical synthesis to incorporate the nonnative cross-links, which is not a scalable synthetic approach. To address this issue, we demonstrate proof of principle for the semisynthesis of insulin analogues bearing nonnative A6-A11 cystine isosteres. The key feature of our synthetic strategy involves the use of several biosynthetically derived peptide precursors which can be produced at scale cost-effectively and a small, chemically synthesised A6-A11 macrocyclic lactam fragment. Although the assembled A6-A11 lactam insulin possesses poor biological activity in vitro, our synthetic strategy can be applied to other disulfide mimetics that have been shown to improve thermal stability without significantly affecting activity and structure. Moreover, we envisage that this new semisynthetic approach will underpin a new generation of hyperstable proteomimetics.
Keywords: cystine mimetic; diabetes; disulfide bond; insulin; peptide synthesis; semisynthesis.
© 2023 The Authors. Journal of Peptide Science published by European Peptide Society and John Wiley & Sons Ltd.
Figures




References
-
- IDF Diabetes Atlas. 10thed. International Diabetes Federation; 2021.
-
- Brayden DJ. The centenary of the discovery of insulin: an update on the quest for oral delivery. Front Drug Deliv. 2021;1. doi: 10.3389/fddev.2021.726675 - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
