Global diversity and antimicrobial resistance of typhoid fever pathogens: Insights from a meta-analysis of 13,000 Salmonella Typhi genomes
- PMID: 37697804
- PMCID: PMC10506625
- DOI: 10.7554/eLife.85867
Global diversity and antimicrobial resistance of typhoid fever pathogens: Insights from a meta-analysis of 13,000 Salmonella Typhi genomes
Abstract
Background: The Global Typhoid Genomics Consortium was established to bring together the typhoid research community to aggregate and analyse Salmonella enterica serovar Typhi (Typhi) genomic data to inform public health action. This analysis, which marks 22 years since the publication of the first Typhi genome, represents the largest Typhi genome sequence collection to date (n=13,000).
Methods: This is a meta-analysis of global genotype and antimicrobial resistance (AMR) determinants extracted from previously sequenced genome data and analysed using consistent methods implemented in open analysis platforms GenoTyphi and Pathogenwatch.
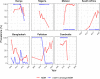
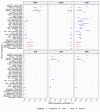
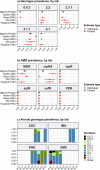
Results: Compared with previous global snapshots, the data highlight that genotype 4.3.1 (H58) has not spread beyond Asia and Eastern/Southern Africa; in other regions, distinct genotypes dominate and have independently evolved AMR. Data gaps remain in many parts of the world, and we show the potential of travel-associated sequences to provide informal 'sentinel' surveillance for such locations. The data indicate that ciprofloxacin non-susceptibility (>1 resistance determinant) is widespread across geographies and genotypes, with high-level ciprofloxacin resistance (≥3 determinants) reaching 20% prevalence in South Asia. Extensively drug-resistant (XDR) typhoid has become dominant in Pakistan (70% in 2020) but has not yet become established elsewhere. Ceftriaxone resistance has emerged in eight non-XDR genotypes, including a ciprofloxacin-resistant lineage (4.3.1.2.1) in India. Azithromycin resistance mutations were detected at low prevalence in South Asia, including in two common ciprofloxacin-resistant genotypes.
Conclusions: The consortium's aim is to encourage continued data sharing and collaboration to monitor the emergence and global spread of AMR Typhi, and to inform decision-making around the introduction of typhoid conjugate vaccines (TCVs) and other prevention and control strategies.
Funding: No specific funding was awarded for this meta-analysis. Coordinators were supported by fellowships from the European Union (ZAD received funding from the European Union's Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement No 845681), the Wellcome Trust (SB, Wellcome Trust Senior Fellowship), and the National Health and Medical Research Council (DJI is supported by an NHMRC Investigator Grant [GNT1195210]).
Keywords: S. enterica serovar typhi; antimicrobial resistance; epidemiology; genomics; global health; infectious disease; microbiology; typhoid conjugate vaccine; typhoid fever.
Plain language summary
Salmonella Typhi (Typhi) is a type of bacteria that causes typhoid fever. More than 110,000 people die from this disease each year, predominantly in areas of sub-Saharan Africa and South Asia with limited access to safe water and sanitation. Clinicians use antibiotics to treat typhoid fever, but scientists worry that the spread of antimicrobial-resistant Typhi could render the drugs ineffective, leading to increased typhoid fever mortality. The World Health Organization has prequalified two vaccines that are highly effective in preventing typhoid fever and may also help limit the emergence and spread of resistant Typhi. In low resource settings, public health officials must make difficult trade-off decisions about which new vaccines to introduce into already crowded immunization schedules. Understanding the local burden of antimicrobial-resistant Typhi and how it is spreading could help inform their actions. The Global Typhoid Genomics Consortium analyzed 13,000 Typhi genomes from 110 countries to provide a global overview of genetic diversity and antimicrobial-resistant patterns. The analysis showed great genetic diversity of the different strains between countries and regions. For example, the H58 Typhi variant, which is often drug-resistant, has spread rapidly through Asia and Eastern and Southern Africa, but is less common in other regions. However, distinct strains of other drug-resistant Typhi have emerged in other parts of the world. Resistance to the antibiotic ciprofloxacin was widespread and accounted for over 85% of cases in South Africa. Around 70% of Typhi from Pakistan were extensively drug-resistant in 2020, but these hard-to-treat variants have not yet become established elsewhere. Variants that are resistant to both ciprofloxacin and ceftriaxone have been identified, and azithromycin resistance has also appeared in several different variants across South Asia. The Consortium’s analyses provide valuable insights into the global distribution and transmission patterns of drug-resistant Typhi. Limited genetic data were available fromseveral regions, but data from travel-associated cases helped fill some regional gaps. These findings may help serve as a starting point for collective sharing and analyses of genetic data to inform local public health action. Funders need to provide ongoing supportto help fill global surveillance data gaps.
© 2023, Carey et al.
Conflict of interest statement
MC, ZD, DI, AA, MA, MC, KC, JC, BH, KK, MM, CP, SV, HW, AA, AA, SA, JA, PA, BB, AB, JC, Kd, AD, Jd, PD, CD, SD, ME, LF, DG, GG, MG, AG, CG, MG, RH, RH, YH, JH, OI, JI, JJ, CJ, DJ, JJ, GK, AK, AK, AK, SK, RK, RK, AL, ML, RL, SL, GM, TM, CM, AM, GN, SN, SN, TN, SN, EN, IO, SP, AP, FQ, FQ, SR, SR, DR, PR, RR, TR, JR, SS, SS, KS, MS, JS, JS, VS, JS, RS, SS, MS, AS, AS, KT, DT, AT, MT, MT, RT, NT, ST, KV, MV, BV, FW, JW, GD, SA, JK, DA, SB, KH No competing interests declared, NF NAF chairs the Wellcome Surveillance and Epidemiology of Drug Resistant Infections (SEDRIC) group, which has a focus on antimicrobial resistance. This could be perceived as relevant although not a direct conflict, IB IB has consulted to BlueDot and the NHL Players' Association, AP AJP is chair of the UK Department of Health and Social Care's (DHSC) Joint Committee on Vaccination and Immunisation (JCVI) but does not take part in the JCVI COVID-19 committee. He was a member of WHO SAGE until 2022. AJPs employer, Oxford University has entered into a partnership with AstraZeneca for development of a COVID-19 vaccine. AJP has provided advice to Shionogi & Co., Ltd on development of a COVID19 vaccine
Figures




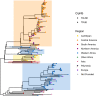

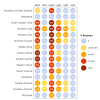


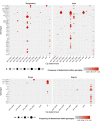


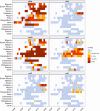




Update of
References
-
- Achtman M, Van den Broeck F, Cooper KK, Lemey P, Parker CT, Zhou Z, ATCC14028s Study Group Genomic population structure associated with repeated escape of Salmonella enterica ATCC14028s from the laboratory into nature. PLOS Genetics. 2021;17:e1009820. doi: 10.1371/journal.pgen.1009820. - DOI - PMC - PubMed
-
- Argimón S, Nagaraj G, Shamanna V, Sravani D, Vasanth AK, Prasanna A, Poojary A, Bari AK, Underwood A, Kekre M, Baker S, Aanensen DM, Lingegowda RK. Circulation of third-generation cephalosporin resistant Salmonella Typhi in Mumbai, India. Clinical Infectious Diseases. 2021a;74:2234–2237. doi: 10.1093/cid/ciab897. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 TW009237/TW/FIC NIH HHS/United States
- BBS/E/F/00PR10348/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- F30 AI156973/AI/NIAID NIH HHS/United States
- BB/R012504/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- U01 AI062563/AI/NIAID NIH HHS/United States
- MR/TOO5033/1/MRC_/Medical Research Council/United Kingdom
- 206545/7/17/Z/WT_/Wellcome Trust/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- 106158/7/14/Z/WT_/Wellcome Trust/United Kingdom
- 001/WHO_/World Health Organization/International
- R01 AI121378/AI/NIAID NIH HHS/United States
- U19 AI110820/AI/NIAID NIH HHS/United States
- R01 AI099525/AI/NIAID NIH HHS/United States
- 206194/WT_/Wellcome Trust/United Kingdom
- MR/L00464X//MRC_/Medical Research Council/United Kingdom

