Semi-supervised mixture multi-source exchangeability model for leveraging real-world data in clinical trials
- PMID: 37697901
- PMCID: PMC11247180
- DOI: 10.1093/biostatistics/kxad024
Semi-supervised mixture multi-source exchangeability model for leveraging real-world data in clinical trials
Erratum in
-
Correction.Biostatistics. 2024 Dec 31;26(1):kxae029. doi: 10.1093/biostatistics/kxae029. Biostatistics. 2024. PMID: 39186534 Free PMC article. No abstract available.
Abstract
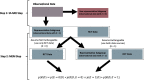
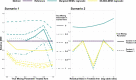
The traditional trial paradigm is often criticized as being slow, inefficient, and costly. Statistical approaches that leverage external trial data have emerged to make trials more efficient by augmenting the sample size. However, these approaches assume that external data are from previously conducted trials, leaving a rich source of untapped real-world data (RWD) that cannot yet be effectively leveraged. We propose a semi-supervised mixture (SS-MIX) multisource exchangeability model (MEM); a flexible, two-step Bayesian approach for incorporating RWD into randomized controlled trial analyses. The first step is a SS-MIX model on a modified propensity score and the second step is a MEM. The first step targets a representative subgroup of individuals from the trial population and the second step avoids borrowing when there are substantial differences in outcomes among the trial sample and the representative observational sample. When comparing the proposed approach to competing borrowing approaches in a simulation study, we find that our approach borrows efficiently when the trial and RWD are consistent, while mitigating bias when the trial and external data differ on either measured or unmeasured covariates. We illustrate the proposed approach with an application to a randomized controlled trial investigating intravenous hyperimmune immunoglobulin in hospitalized patients with influenza, while leveraging data from an external observational study to supplement a subgroup analysis by influenza subtype.
Keywords: Bayesian model averaging; Causal inference; Influenza; Propensity scores; Real-world data.
© The Author(s) 2023. Published by Oxford University Press. All rights reserved. [br]For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
None declared.
Figures


References
-
- Bareinboim E., Pearl J. (2013). A general algorithm for deciding transportability of experimental results. Journal of Causal Inference 1, 107–134.
-
- Chen W. C., Wang C., Li H., Lu N., Tiwari R., Xu Y., Yue L. Q. (2020). Propensity score-integrated composite likelihood approach for augmenting the control arm of a randomized controlled trial by incorporating real-world data. Journal of Biopharmaceutical Statistics 30, 508–520. - PubMed
-
- Wang Chenguang (2021). psrwe: PS-integrated methods for incorporating RWE in clinical studies. R package version 2.2. https://github.com/olssol/psrwe.
-
- Chib S. (1996). Calculating posterior distributions and modal estimates in Markov mixture models. Journal of Econometrics 75, 79–97.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

