Farnesoid X receptor enhances epithelial ACE2 expression and inhibits virally induced IL-6 secretion: implications for intestinal symptoms of SARS-CoV-2
- PMID: 37697930
- PMCID: PMC10887846
- DOI: 10.1152/ajpgi.00099.2023
Farnesoid X receptor enhances epithelial ACE2 expression and inhibits virally induced IL-6 secretion: implications for intestinal symptoms of SARS-CoV-2
Abstract
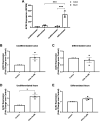
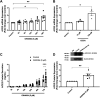
Intestinal inflammation and diarrhea are often associated with SARS-CoV-2 infection. The angiotensin converting enzyme 2 (ACE2) receptor plays a key role in SARS-CoV-2 pathogenesis, facilitating entry of the virus into epithelial cells, while also regulating mucosal inflammatory responses. Here, we investigated roles for the nuclear bile acid receptor farnesoid X receptor (FXR) in regulating ACE2 expression and virally mediated inflammatory responses in intestinal epithelia. Human colonic or ileal enteroids and cultured T84 and Caco-2 monolayers were treated with the FXR agonists, obeticholic acid (OCA) or GW4064, or infected with live SARS-CoV-2 (2019-nCoV/USA_WA1/2020). Changes in mRNA, protein, or secreted cytokines were measured by qPCR, Western blotting, and ELISA. Treatment of undifferentiated colonic or ileal enteroids with OCA increased ACE2 mRNA by 2.1 ± 0.4-fold (n = 3; P = 0.08) and 2.3 ± 0.2-fold (n = 3; P < 0.05), respectively. In contrast, ACE2 expression in differentiated enteroids was not significantly altered. FXR activation in cultured epithelial monolayers also upregulated ACE2 mRNA, accompanied by increases in ACE2 expression and secretion. Further experiments revealed FXR activation to inhibit IL-6 release from both Caco-2 cells infected with SARS-CoV-2 and T84 cells treated with the viral mimic, polyinosinic:polycytidylic acid, by 46 ± 12% (n = 3, P < 0.05) and 35 ± 6% (n = 8; P < 0.01), respectively. By virtue of its ability to modulate epithelial ACE2 expression and inhibit virus-mediated proinflammatory cytokine release, FXR represents a promising target for the development of new approaches to prevent intestinal manifestations of SARS-CoV-2.NEW & NOTEWORTHY Activation of the nuclear bile acid receptor, farnesoid X receptor (FXR), specifically upregulates ACE2 expression in undifferentiated colonic epithelial cells and inhibits virus-induced proinflammatory cytokine release. By virtue of these actions FXR represents a promising target for the development of new approaches to prevent intestinal manifestations of SARS-CoV-2 infection.
Keywords: ACE2; SARS-CoV-2; bile acid; farnesoid X receptor; intestinal epithelium.
Conflict of interest statement
Luciano Adorini is a consultant of Intercept Pharmaceuticals. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
Figures



References
-
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395: 497–506, 2020. doi: 10.1016/S0140-6736(20)30183-5. - DOI - PMC - PubMed
-
- Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395: 507–513, 2020. doi: 10.1016/S0140-6736(20)30211-7. - DOI - PMC - PubMed
-
- Effenberger M, Grabherr F, Mayr L, Schwaerzler J, Nairz M, Seifert M, Hilbe R, Seiwald S, Scholl-Buergi S, Fritsche G, Bellmann-Weiler R, Weiss G, Müller T, Adolph TE, Tilg H. Faecal calprotectin indicates intestinal inflammation in COVID-19. Gut 69: 1543–1544, 2020. doi: 10.1136/gutjnl-2020-321388. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

