Two Days Versus Five Days of Postoperative Antibiotics for Complex Appendicitis: Cost Analysis of a Randomized, Noninferiority Trial
- PMID: 37698025
- PMCID: PMC10997181
- DOI: 10.1097/SLA.0000000000006089
Two Days Versus Five Days of Postoperative Antibiotics for Complex Appendicitis: Cost Analysis of a Randomized, Noninferiority Trial
Abstract
Objective: To compare costs for 2 days versus 5 days of postoperative antibiotics within the antibiotics after an aPPendectomy In Complex appendicitis trial.Background:Recent studies suggest that restrictive antibiotic use leads to a significant reduction in hospital stays without compromising patient safety. Its potential effect on societal costs remains underexplored.
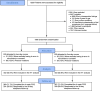
Methods: This was a pragmatic, open-label, multicenter clinical trial powered for noninferiority. Patients with complex appendicitis (age ≥ 8 years) were randomly allocated to 2 days or 5 days of intravenous antibiotics after appendectomy. Patient inclusion lasted from June 2017 to June 2021 in 15 Dutch hospitals. The final follow-up was on September 1, 2021. The primary trial endpoint was a composite endpoint of infectious complications and mortality within 90 days. In the present study, the main outcome measures were overall societal costs (comprising direct health care costs and costs related to productivity loss) and cost-effectiveness. Direct health care costs were recorded based on data in the electronic patient files, complemented by a telephone follow-up at 90 days. In addition, data on loss of productivity were acquired through the validated Productivity Cost Questionnaire at 4 weeks after surgery. Cost estimates were based on prices for the year 2019.
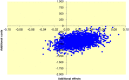
Results: In total, 1005 patients were evaluated in the "intention-to-treat" analysis: 502 patients were allocated to the 2-day group and 503 to the 5-day group. The mean difference in overall societal costs was - €625 (95% CI: -€ 958 to -€ 278) to the advantage of the 2-day group. This difference was largely explained by reduced hospital stay. Productivity losses were similar between the study groups. Restricting postoperative antibiotics to 2 days was cost-effective, with estimated cost savings of €31,117 per additional infectious complication.
Conclusions: Two days of postoperative antibiotics for complex appendicitis results in a statistically significant and relevant cost reduction, as compared with 5 days. Findings apply to laparoscopic appendectomy in a well-resourced health care setting.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors report no conflicts of interest.
Figures

References
-
- Ferris M, Quan S, Kaplan BS, et al. . The global incidence of appendicitis: a systematic review of population-based studies. Ann Surg. 2017;266:237–241. - PubMed
-
- Bhangu A, Soreide K, Di Saverio S, et al. . Acute appendicitis: modern understanding of pathogenesis, diagnosis, and management. Lancet. 2015;386:1278–1287. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

