Development of Electronic Health Record-Based Machine Learning Models to Predict Barrett's Esophagus and Esophageal Adenocarcinoma Risk
- PMID: 37698203
- PMCID: PMC10584285
- DOI: 10.14309/ctg.0000000000000637
Development of Electronic Health Record-Based Machine Learning Models to Predict Barrett's Esophagus and Esophageal Adenocarcinoma Risk
Abstract
Introduction: Screening for Barrett's esophagus (BE) is suggested in those with risk factors, but remains underutilized. BE/esophageal adenocarcinoma (EAC) risk prediction tools integrating multiple risk factors have been described. However, accuracy remains modest (area under the receiver-operating curve [AUROC] ≤0.7), and clinical implementation has been challenging. We aimed to develop machine learning (ML) BE/EAC risk prediction models from an electronic health record (EHR) database.
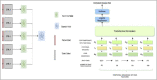
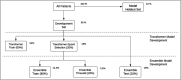
Methods: The Clinical Data Analytics Platform, a deidentified EHR database of 6 million Mayo Clinic patients, was used to predict BE and EAC risk. BE and EAC cases and controls were identified using International Classification of Diseases codes and augmented curation (natural language processing) techniques applied to clinical, endoscopy, laboratory, and pathology notes. Cases were propensity score matched to 5 independent randomly selected control groups. An ensemble transformer-based ML model architecture was used to develop predictive models.
Results: We identified 8,476 BE cases, 1,539 EAC cases, and 252,276 controls. The BE ML transformer model had an overall sensitivity, specificity, and AUROC of 76%, 76%, and 0.84, respectively. The EAC ML transformer model had an overall sensitivity, specificity, and AUROC of 84%, 70%, and 0.84, respectively. Predictors of BE and EAC included conventional risk factors and additional novel factors, such as coronary artery disease, serum triglycerides, and electrolytes.
Discussion: ML models developed on an EHR database can predict incident BE and EAC risk with improved accuracy compared with conventional risk factor-based risk scores. Such a model may enable effective implementation of a minimally invasive screening technology.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of The American College of Gastroenterology.
Conflict of interest statement
Figures



References
-
- Asge Standards Of Practice C, Qumseya B, Sultan S, et al. ASGE guideline on screening and surveillance of Barrett's esophagus. Gastrointest Endosc 2019;90(3):335–59.e2. - PubMed
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

