The Alzheimer's Disease Neuroimaging Initiative in the era of Alzheimer's disease treatment: A review of ADNI studies from 2021 to 2022
- PMID: 37698424
- PMCID: PMC10841343
- DOI: 10.1002/alz.13449
The Alzheimer's Disease Neuroimaging Initiative in the era of Alzheimer's disease treatment: A review of ADNI studies from 2021 to 2022
Abstract
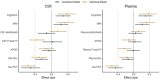
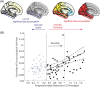
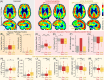
The Alzheimer's Disease Neuroimaging Initiative (ADNI) aims to improve Alzheimer's disease (AD) clinical trials. Since 2006, ADNI has shared clinical, neuroimaging, and cognitive data, and biofluid samples. We used conventional search methods to identify 1459 publications from 2021 to 2022 using ADNI data/samples and reviewed 291 impactful studies. This review details how ADNI studies improved disease progression understanding and clinical trial efficiency. Advances in subject selection, detection of treatment effects, harmonization, and modeling improved clinical trials and plasma biomarkers like phosphorylated tau showed promise for clinical use. Biomarkers of amyloid beta, tau, neurodegeneration, inflammation, and others were prognostic with individualized prediction algorithms available online. Studies supported the amyloid cascade, emphasized the importance of neuroinflammation, and detailed widespread heterogeneity in disease, linked to genetic and vascular risk, co-pathologies, sex, and resilience. Biological subtypes were consistently observed. Generalizability of ADNI results is limited by lack of cohort diversity, an issue ADNI-4 aims to address by enrolling a diverse cohort.
Keywords: Alzheimer's Disease Neuroimaging Initiative; Alzheimer's disease clinical trials; Alzheimer's disease subtypes; amyloid; cerebrovascular disease; co-pathologies; diagnosis; disease progression; generalizability; neurodegeneration; neuroinflammation; plasma biomarkers; prediction; resilience; tau.
© 2023 The Authors. Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Dr. Ashford, Dr. Beckett, Dr. Jack, Dr. Miller, Dr. Morris, Dr. Nho, Dr. Okonkwo, Dr. Perrin, Dr. Toga, Dr. Tosun, and Dr. Veitch have no conflicts to declare. Dr. Aisen has research grants from NIH, Lilly, and Eisai, and consults with Merck, Roche, Genentech, Abbvie, Biogen, ImmunoBrain Checkpoint, and Arrowhead. Dr. Green has received compensation for advising the following companies: Allelica, Atria, Fabric, Genome Web, Genomic Life, Grail, Verily, and VinBigData and is co‐founder of Genome Medical and Nurture Genomics. Dr. Harvey serves as a Statistical Advisor for
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

