Pneumococcal infections in children with sickle cell disease before and after pneumococcal conjugate vaccines
- PMID: 37698500
- PMCID: PMC10660014
- DOI: 10.1182/bloodadvances.2022009643
Pneumococcal infections in children with sickle cell disease before and after pneumococcal conjugate vaccines
Abstract
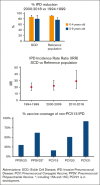
Children with sickle cell disease (SCD) are at increased risk of invasive pneumococcal disease (IPD). Over 25 years, the Georgia Emerging Infections Program/Centers for Disease Control and Prevention Active Bacterial Core Surveillance network identified 104 IPD episodes among 3707 children with hemoglobin SS (HbSS) or HbSC aged <10 years, representing 6% of IPD in Black or African American children residing in Metropolitan Atlanta (reference population). Children with IPD and HbSS/SC were older than those with IPD in the reference population (P < .001). From 1994-1999 to 2010-2018, IPD declined by 87% in children with HbSS aged 0 to 4 years, and by 80% in those aged 5 to 9 years. However, IPD incidence rate ratios when comparing children with SCD with the reference population increased from 20.2 to 29.2 over these periods. Among children with HbSS and IPD, death declined from 14% to 3% after 2002, and meningitis declined from 16% to 8%. Penicillin resistance was more prevalent in children with SCD before 7-valent pneumococcal conjugate vaccine (PCV7) licensure. After 2010, all IPD serotypes were not included in the 13-valent PCV (PCV13). Within 3 years of vaccination, the effectiveness of the 23-valent pneumococcal polysaccharide vaccine (PPSV23) against non-PCV13 serotypes included in PPSV23 plus 15A/15C was 92% (95% confidence interval, 40.8- 99.0, P = .014; indirect-cohort effect adjusted for age and hydroxyurea). PPSV23 would cover 62% of non-PCV13 serotype IPD in children with SCD, whereas PCV15, PCV20, and PCV21/V116 (in development) could cover 16%, 51%, and 92%, respectively. Although less frequent, IPD remains a life-threatening risk in children with SCD. Effective vaccines with broader coverage could benefit these children.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: I.Y. has received funding to her institution to conduct clinical research unrelated to this manuscript from the Gates Foundation, Centers for Disease Control and Prevention, National Institutes of Health, Moderna, and Pfizer; and consults for Merck and Sanofi-Pasteur. The remaining authors declare no competing financial interests.
Figures
References
-
- Ware RE, de Montalembert M, Tshilolo L, Abboud MR. Sickle cell disease. Lancet. 2017;390(10091):311–323. - PubMed
-
- Lane PA, O'Connell JL, Lear JL, et al. Functional asplenia in hemoglobin SC disease. Blood. 1995;85(8):2238–2244. - PubMed
-
- Powars D, Overturf G, Weiss J, Lee S, Chan L. Pneumococcal septicemia in children with sickle cell anemia. Changing trend of survival. JAMA. 1981;245(18):1839–1842. - PubMed
-
- Gaston MH, Verter JI, Woods G, et al. Prophylaxis with oral penicillin in children with sickle cell anemia. A randomized trial. N Engl J Med. 1986;314(25):1593–1599. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical