Molecular consequences of peripheral Influenza A infection on cell populations in the murine hypothalamus
- PMID: 37698546
- PMCID: PMC10497288
- DOI: 10.7554/eLife.87515
Molecular consequences of peripheral Influenza A infection on cell populations in the murine hypothalamus
Abstract
Infection with Influenza A virus (IAV) causes the well-known symptoms of the flu, including fever, loss of appetite, and excessive sleepiness. These responses, mediated by the brain, will normally disappear once the virus is cleared from the system, but a severe respiratory virus infection may cause long-lasting neurological disturbances. These include encephalitis lethargica and narcolepsy. The mechanisms behind such long lasting changes are unknown. The hypothalamus is a central regulator of the homeostatic response during a viral challenge. To gain insight into the neuronal and non-neuronal molecular changes during an IAV infection, we intranasally infected mice with an H1N1 virus and extracted the brain at different time points. Using single-nucleus RNA sequencing (snRNA-seq) of the hypothalamus, we identify transcriptional effects in all identified cell populations. The snRNA-seq data showed the most pronounced transcriptional response at 3 days past infection, with a strong downregulation of genes across all cell types. General immune processes were mainly impacted in microglia, the brain resident immune cells, where we found increased numbers of cells expressing pro-inflammatory gene networks. In addition, we found that most neuronal cell populations downregulated genes contributing to the energy homeostasis in mitochondria and protein translation in the cytosol, indicating potential reduced cellular and neuronal activity. This might be a preventive mechanism in neuronal cells to avoid intracellular viral replication and attack by phagocytosing cells. The change of microglia gene activity suggest that this is complemented by a shift in microglia activity to provide increased surveillance of their surroundings.
Keywords: hypothalamus; immunology; inflammation; influenza a virus; mouse; neurons; neuroscience; non-neuronal cells; olfactory bulb; snRNA sequencing; viruses.
Plain language summary
When you are ill, your behaviour changes. You sleep more, eat less and are less likely to go out and be active. This behavioural change is called the ‘sickness response’ and is believed to help the immune system fight infection. An area of the brain called the hypothalamus helps to regulate sleep and appetite. Previous research has shown that when humans are ill, the immune system sends signals to the hypothalamus, likely initiating the sickness response. However, it was not clear which brain cells in the hypothalamus are involved in the response and how long after infection the brain returns to its normal state. To better understand the sickness response, Lemcke et al. infected mice with influenza then extracted and analysed brain tissue at different timepoints. The experiments showed that the major changes to gene expression in the hypothalamus early during an influenza infection are not happening in neurons – the cells in the brain that transmit electrical signals and usually control behaviour. Instead, it is cells called glia – which provide support and immune protection to the neurons – that change during infection. The findings suggest that these cells prepare to protect the neurons from influenza should the virus enter the brain. Lemcke et al. also found that the brain takes a long time to go back to normal after an influenza infection. In infected mice, molecular changes in brain cells could be detected even after the influenza infection had been cleared from the respiratory system. In the future, these findings may help to explain why some people take longer than others to fully recover from viral infections such as influenza and aid development of medications that speed up recovery.
© 2023, Lemcke et al.
Conflict of interest statement
RL, CE, NB, KE, AT, TP, JC, BK No competing interests declared
Figures















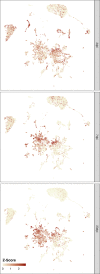


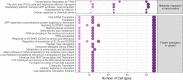


Update of
- doi: 10.1101/2023.03.06.530999
- doi: 10.7554/eLife.87515.1
- doi: 10.7554/eLife.87515.2
Similar articles
-
Influenza A Virus (H1N1) Infection Induces Microglial Activation and Temporal Dysbalance in Glutamatergic Synaptic Transmission.mBio. 2021 Oct 26;12(5):e0177621. doi: 10.1128/mBio.01776-21. Epub 2021 Oct 26. mBio. 2021. PMID: 34700379 Free PMC article.
-
Non-hydrolyzed in digestive tract and blood natural L-carnosine peptide ("bioactivated Jewish penicillin") as a panacea of tomorrow for various flu ailments: signaling activity attenuating nitric oxide (NO) production, cytostasis, and NO-dependent inhibition of influenza virus replication in macrophages in the human body infected with the virulent swine influenza A (H1N1) virus.J Basic Clin Physiol Pharmacol. 2013;24(1):1-26. doi: 10.1515/jbcpp-2012-0037. J Basic Clin Physiol Pharmacol. 2013. PMID: 23425625 Review.
-
Microglia Activate Early Antiviral Responses upon Herpes Simplex Virus 1 Entry into the Brain to Counteract Development of Encephalitis-Like Disease in Mice.J Virol. 2022 Mar 23;96(6):e0131121. doi: 10.1128/JVI.01311-21. Epub 2022 Jan 19. J Virol. 2022. PMID: 35045263 Free PMC article.
-
Long-Term Neuroinflammation Induced by Influenza A Virus Infection and the Impact on Hippocampal Neuron Morphology and Function.J Neurosci. 2018 Mar 21;38(12):3060-3080. doi: 10.1523/JNEUROSCI.1740-17.2018. Epub 2018 Feb 27. J Neurosci. 2018. PMID: 29487124 Free PMC article.
-
Narcolepsy and H1N1 influenza immunology a decade later: What have we learned?Front Immunol. 2022 Oct 12;13:902840. doi: 10.3389/fimmu.2022.902840. eCollection 2022. Front Immunol. 2022. PMID: 36311717 Free PMC article. Review.
Cited by
-
Does COVID-19 Infection Continue to Affect Self-Reported and Objective Sleep? A Longitudinal Study of Good Sleepers.Nat Sci Sleep. 2025 Jun 19;17:1421-1430. doi: 10.2147/NSS.S522439. eCollection 2025. Nat Sci Sleep. 2025. PMID: 40552070 Free PMC article.
-
Glia: the cellular glue that binds circadian rhythms and sleep.Sleep. 2025 Mar 11;48(3):zsae314. doi: 10.1093/sleep/zsae314. Sleep. 2025. PMID: 39812780 Free PMC article. Review.
References
-
- Ahmed SS, Schur PH, MacDonald NE, Steinman L. Narcolepsy, 2009 A(H1N1) pandemic influenza, and pandemic influenza vaccinations: what is known and unknown about the neurological disorder, the role for autoimmunity, and vaccine adjuvants. Journal of Autoimmunity. 2014;50:1–11. doi: 10.1016/j.jaut.2014.01.033. - DOI - PubMed
-
- Alexa A. Enrichment analysis for gene Ontology. R package version 2.48.0topGO 2022
Publication types
MeSH terms
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

