Direct comparison and reproducibility of two segmentation methods for multicompartment dosimetry: round robin study on radioembolization treatment planning in hepatocellular carcinoma
- PMID: 37698645
- PMCID: PMC10684706
- DOI: 10.1007/s00259-023-06416-9
Direct comparison and reproducibility of two segmentation methods for multicompartment dosimetry: round robin study on radioembolization treatment planning in hepatocellular carcinoma
Abstract
Purpose: Investigate reproducibility of two segmentation methods for multicompartment dosimetry, including normal tissue absorbed dose (NTAD) and tumour absorbed dose (TAD), in hepatocellular carcinoma patients treated with yttrium-90 (90Y) glass microspheres.
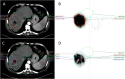
Methods: TARGET was a retrospective investigation in 209 patients with < 10 tumours per lobe and at least one tumour ≥ 3 cm ± portal vein thrombosis. Dosimetry was compared using two distinct segmentation methods: anatomic (CT/MRI-based) and count threshold-based on pre-procedural 99mTc-MAA SPECT. In a round robin substudy in 20 patients with ≤ 5 unilobar tumours, the inter-observer reproducibility of eight reviewers was evaluated by computing reproducibility coefficient (RDC) of volume and absorbed dose for whole liver, whole liver normal tissue, perfused normal tissue, perfused liver, total perfused tumour, and target lesion. Intra-observer reproducibility was based on second assessments in 10 patients ≥ 2 weeks later.
Results: 99mTc-MAA segmentation calculated higher absorbed doses compared to anatomic segmentation (n = 209), 43.9% higher for TAD (95% limits of agreement [LoA]: - 49.0%, 306.2%) and 21.3% for NTAD (95% LoA: - 67.6%, 354.0%). For the round robin substudy (n = 20), inter-observer reproducibility was better for anatomic (RDC range: 1.17 to 3.53) than 99mTc-MAA SPECT segmentation (1.29 to 7.00) and similar between anatomic imaging modalities (CT: 1.09 to 3.56; MRI: 1.24 to 3.50). Inter-observer reproducibility was better for larger volumes. Perfused normal tissue volume RDC was 1.95 by anatomic and 3.19 by 99mTc-MAA SPECT, with corresponding absorbed dose RDC 1.46 and 1.75. Total perfused tumour volume RDC was higher, 2.92 for anatomic and 7.0 by 99mTc-MAA SPECT with corresponding absorbed dose RDC of 1.84 and 2.78. Intra-observer variability was lower for perfused NTAD (range: 14.3 to 19.7 Gy) than total perfused TAD (range: 42.8 to 121.4 Gy).
Conclusion: Anatomic segmentation-based dosimetry, versus 99mTc-MAA segmentation, results in lower absorbed doses with superior reproducibility. Higher volume compartments, such as normal tissue versus tumour, exhibit improved reproducibility.
Trial registration: NCT03295006.
Keywords: Dosimetry; Hepatocellular carcinoma; Radioembolization; Yttrium-90.
© 2023. The Author(s).
Conflict of interest statement
Marnix Lam, MD, PhD, is a consultant for Boston Scientific, Terumo, and Quirem Medical. He receives research support from Boston Scientific, Terumo, and Quirem Medical. The UMC Utrecht receives royalties from Quirem Medical.
Etienne Garin, MD, PhD, serves as a consultant for Boston Scientific.
Xavier Palard-Novello, MD, PhD: none.
Armeen Mahvash, MD, is a consultant for Boston Scientific, Sirtex Medical, and ABK Biomedical. He received research support from Boston Scientific, Sirtex Medical, ABK Biomedical, and Siemens Healthineers.
Cheenu Kappadath, PhD, is a consultant for Boston Scientific, Sirtex Medical, and Terumo Medical. He receives research support from Boston Scientific, Sirtex Medical, and Terumo Medical.
Paul Haste, MD, is a consultant for Boston Scientific.
Mark Tann, MD: none.
Ken Herrmann, MD, reports personal fees from Bayer, personal fees and others from Sofie Biosciences, personal fees from SIRTEX, non-financial support from ABX, personal fees from Adacap, personal fees from Curium, personal fees from Endocyte, grants and personal fees from BTG, personal fees from IPSEN, personal fees from Siemens Healthineers, personal fees from GE Healthcare, personal fees from Amgen, personal fees from Novartis, personal fees from ymabs, personal fees from Aktis Oncology, personal fees from Theragnostics, personal fees from Pharma15, and outside the submitted work.
Francesco Barbato, MD: none.
Brian Geller, MD, is a consultant for Boston Scientific.
Niklaus Schaefer, MD: none.
Alban Denys, MD, MSc, is a consultant for Cook, Neuwave, and received grand from Johnson and Johnson.
Matthew R. Dreher, PhD, works for Boston Scientific.
Kirk D. Fowers, PhD, works for Boston Scientific.
Riad Salem, MD, is a consultant for Boston Scientific, Astrazeneca, Genentech, Sirtex, Cook, Eisai, Bard, and QED Therapeutics.
Vanessa L. Gates, MS, is a consultant for Boston Scientific.
Figures




References
-
- d'Abadie P, Walrand S, Hesse M, Annet L, Borbath I, Van den Eynde M, et al. Prediction of tumor response and patient outcome after radioembolization of hepatocellular carcinoma using 90Y-PET-computed tomography dosimetry. Nucl Med Commun. 2021;42:747–754. doi: 10.1097/mnm.0000000000001395. - DOI - PubMed
-
- Chan KT, Alessio AM, Johnson GE, Vaidya S, Kwan SW, Monsky W, et al. Prospective trial using internal pair-production positron emission tomography to establish the yttrium-90 radioembolization dose required for response of hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2018;101:358–365. doi: 10.1016/j.ijrobp.2018.01.116. - DOI - PubMed
-
- Kappadath SC, Mikell J, Balagopal A, Baladandayuthapani V, Kaseb A, Mahvash A. Hepatocellular carcinoma tumor dose response after (90)Y-radioembolization with glass microspheres using (90)Y-SPECT/CT-based voxel dosimetry. Int J Radiat Oncol Biol Phys. 2018;102:451–461. doi: 10.1016/j.ijrobp.2018.05.062. - DOI - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

