PLK1 inhibition dampens NLRP3 inflammasome-elicited response in inflammatory disease models
- PMID: 37698938
- PMCID: PMC10617773
- DOI: 10.1172/JCI162129
PLK1 inhibition dampens NLRP3 inflammasome-elicited response in inflammatory disease models
Abstract
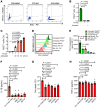
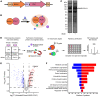
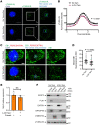
Unabated activation of the NLR family pyrin domain-containing 3 (NLRP3) inflammasome is linked with the pathogenesis of various inflammatory disorders. Polo-like kinase 1 (PLK1) has been widely studied for its role in mitosis. Here, using both pharmacological and genetic approaches, we demonstrate that PLK1 promoted NLRP3 inflammasome activation at cell interphase. Using an unbiased proximity-dependent biotin identification (Bio-ID) screen for the PLK1 interactome in macrophages, we show an enhanced proximal association of NLRP3 with PLK1 upon NLRP3 inflammasome activation. We further confirmed the interaction between PLK1 and NLRP3 and identified the interacting domains. Mechanistically, we show that PLK1 orchestrated the microtubule-organizing center (MTOC) structure and NLRP3 subcellular positioning upon inflammasome activation. Treatment with a selective PLK1 kinase inhibitor suppressed IL-1β production in in vivo inflammatory models, including LPS-induced endotoxemia and monosodium urate-induced peritonitis in mice. Our results uncover a role of PLK1 in regulating NLRP3 inflammasome activation during interphase and identify pharmacological inhibition of PLK1 as a potential therapeutic strategy for inflammatory diseases with excessive NLRP3 inflammasome activation.
Keywords: Cell Biology; Cellular immune response; Cytoskeleton; Immunology.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
- RE/18/1/34212/BHF_/British Heart Foundation/United Kingdom
- FS/14/28/30713/BHF_/British Heart Foundation/United Kingdom
- PG/17/69/33229/BHF_/British Heart Foundation/United Kingdom
- DH_/Department of Health/United Kingdom
- FS/20/19/34976/BHF_/British Heart Foundation/United Kingdom
- FS/SBSRF/22/31036/BHF_/British Heart Foundation/United Kingdom
- 108045/Z/15/Z/WT_/Wellcome Trust/United Kingdom
- CRI_CORE_BIOIN_2324/CRUK_/Cancer Research UK/United Kingdom
- SP/F/22/150038/BHF_/British Heart Foundation/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- FS/16/53/32729/BHF_/British Heart Foundation/United Kingdom
- CH/10/001/27642/BHF_/British Heart Foundation/United Kingdom
- RG/16/8/32388/BHF_/British Heart Foundation/United Kingdom
- FS/18/19/33371/BHF_/British Heart Foundation/United Kingdom
- FS/17/5/32531/BHF_/British Heart Foundation/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

