Safety and Immunogenicity of a Revaccination With a Respiratory Syncytial Virus Prefusion F Vaccine in Older Adults: A Phase 2b Study
- PMID: 37699064
- PMCID: PMC10873183
- DOI: 10.1093/infdis/jiad321
Safety and Immunogenicity of a Revaccination With a Respiratory Syncytial Virus Prefusion F Vaccine in Older Adults: A Phase 2b Study
Abstract
Background: In the previous (parent) study, 2 doses of different formulations of an investigational vaccine against respiratory syncytial virus (RSVPreF3 OA) were well tolerated and immunogenic in older adults. This multicenter phase 2b extension study assessed safety and immunogenicity of a revaccination (third) dose of the 120 μg RSVPreF3-AS01E formulation.
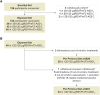
Methods: In total, 122 older adults (60-80 years), previously vaccinated with 2 doses of RSVPreF3-AS01E formulations (containing 30, 60, or 120 μg RSVPreF3 antigen), received an additional 120 μg RSVPreF3-AS01E dose 18 months after dose 2. Vaccine safety was evaluated in all participants up to 6 months and immunogenicity in participants who received 120 μg RSVPreF3-AS01E doses until 1 month after dose 3.
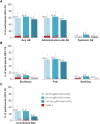
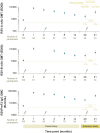
Results: Similar to the parent study, mostly mild-to-moderate solicited adverse events and no vaccine-related serious adverse events or potential immune-mediated disorders were reported. Neutralizing titers and cell-mediated immune responses persisted for 18 months after 2-dose vaccination. Dose 3 increased RSV-specific neutralizing titers against RSV-A and RSV-B and median CD4+ T-cell frequencies. After dose 3, RSV-specific neutralizing titers but not CD4+ T-cell frequencies were below levels detected 1 month after dose 1.
Conclusions: Revaccination with 120 μg RSVPreF3-AS01E 18 months after dose 2 is well tolerated and immunogenic in older adults.
Clinical trials registration: NCT04657198; EudraCT, 2020-000692-21.
Keywords: AS01E; RSV neutralizing titers; RSV vaccine; RSVPreF3; cell-mediated immunity; respiratory syncytial virus.
Plain language summary
Respiratory syncytial virus (RSV) is a common, contagious seasonal virus causing respiratory tract infections. In older adults, RSV can cause serious respiratory illnesses or worsen underlying medical conditions such as chronic diseases of the lungs or heart failure. Severe disease may lead to hospitalization, increased need for oxygen, and ventilatory support. However, several vaccines against RSV in older adults have recently been licensed in the United States and European Union. This study evaluated safety and immune responses after revaccination (third dose) with an adjuvanted vaccine against RSV in older adults aged 60–80 years, who had received 2 doses of the vaccine with a similar adjuvanted formulation in a previous (parent) study. Revaccination was done with the licensed vaccine formulation, which was also selected for further investigation in several phase 3 clinical trials. This study found that immune responses against RSV persisted above prevaccination levels for at least 18 months after the second vaccination in the parent study. The third vaccine dose was well tolerated and recalled the immune responses in older adults. Together with the ongoing confirmatory clinical trials, these results help better characterize this RSV vaccine, in terms of safety and RSV-specific immune responses elicited in older adults.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. I. L. R. reports funding from Icosavax and Virometix to her institution for the conduct of RSV vaccine trials, and from GSK to her institution for conduct of this clinical trial. M. V. R. reports GSK funding to the Leuven University Vaccinology Center for the conduct of the clinical trial. C. Vm. reports GSK funding to her institution for the conduct of the clinical trial as at the time of the execution of the clinical trial she was an investigator; C. Vm. joined GSK after the close of the study at her institution and is currently an employee of GSK; however, she does not hold any shares. C. V. A., N. D. S., B. S., C. V., M. P. D., S. K., and V. H. were employees of GSK at the time of the study conduct. N. D. S., B. S., M. P. D., C. V., S. K., and V. H. hold shares from GSK as part of their past/current employee remuneration. All current/previous employees of GSK declare financial and nonfinancial relationships and activities. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures


Similar articles
-
Immunogenicity and Safety Following 1 Dose of AS01E-Adjuvanted Respiratory Syncytial Virus Prefusion F Protein Vaccine in Older Adults: A Phase 3 Trial.J Infect Dis. 2024 Jul 25;230(1):e102-e110. doi: 10.1093/infdis/jiad546. J Infect Dis. 2024. PMID: 39052726 Free PMC article. Clinical Trial.
-
Efficacy, safety, and immunogenicity of the AS01E-adjuvanted respiratory syncytial virus prefusion F protein vaccine (RSVPreF3 OA) in older adults over three respiratory syncytial virus seasons (AReSVi-006): a multicentre, randomised, observer-blinded, placebo-controlled, phase 3 trial.Lancet Respir Med. 2025 Jun;13(6):517-529. doi: 10.1016/S2213-2600(25)00048-7. Epub 2025 Apr 14. Lancet Respir Med. 2025. PMID: 40245915 Clinical Trial.
-
Safety and Immunogenicity of a Respiratory Syncytial Virus Prefusion F (RSVPreF3) Candidate Vaccine in Older Adults: Phase 1/2 Randomized Clinical Trial.J Infect Dis. 2023 Mar 28;227(6):761-772. doi: 10.1093/infdis/jiac327. J Infect Dis. 2023. PMID: 35904987 Free PMC article. Clinical Trial.
-
Vaccines for Respiratory Syncytial Virus Prevention in Older Adults.Ann Pharmacother. 2024 Dec;58(12):1218-1228. doi: 10.1177/10600280241241049. Epub 2024 Apr 2. Ann Pharmacother. 2024. PMID: 38563554 Review.
-
Respiratory Syncytial Virus Maternal Vaccination in Infants below 6 Months of Age: Meta-Analysis of Safety, Immunogenicity, and Efficacy.Neonatology. 2024;121(3):271-282. doi: 10.1159/000536031. Epub 2024 Jan 29. Neonatology. 2024. PMID: 38286126 Free PMC article.
Cited by
-
A foldon-free prefusion F trimer vaccine for respiratory syncytial virus to reduce off-target immune responses.Nat Microbiol. 2024 Dec;9(12):3254-3267. doi: 10.1038/s41564-024-01860-1. Epub 2024 Nov 20. Nat Microbiol. 2024. PMID: 39567664 Free PMC article.
-
Efficacy and Safety of Respiratory Syncytial Virus (RSV) Prefusion F Protein Vaccine (RSVPreF3 OA) in Older Adults Over 2 RSV Seasons.Clin Infect Dis. 2024 Jun 14;78(6):1732-1744. doi: 10.1093/cid/ciae010. Clin Infect Dis. 2024. PMID: 38253338 Free PMC article. Clinical Trial.
-
Health Technology Assessment del vaccino ricombinante adiuvato contro il virus respiratorio sinciziale (Arexvy®).J Prev Med Hyg. 2024 Jun 30;65(2 Suppl 1):E1-E159. doi: 10.15167/2421-4248/jpmh2024.65.2s1. eCollection 2024 Sep. J Prev Med Hyg. 2024. PMID: 39554593 Free PMC article. Italian. No abstract available.
-
Protecting our future: call for respiratory syncytial virus vaccine trial in infants.Ann Med Surg (Lond). 2024 Jul 17;86(9):4960-4962. doi: 10.1097/MS9.0000000000002385. eCollection 2024 Sep. Ann Med Surg (Lond). 2024. PMID: 39238990 Free PMC article. No abstract available.
-
Respiratory Syncytial Virus Vaccines: Analysis of Pre-Marketing Clinical Trials for Immunogenicity in the Population over 50 Years of Age.Vaccines (Basel). 2024 Mar 25;12(4):353. doi: 10.3390/vaccines12040353. Vaccines (Basel). 2024. PMID: 38675736 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

