Fluoxetine restrains allergic inflammation by targeting an FcɛRI-ATP positive feedback loop in mast cells
- PMID: 37699080
- PMCID: PMC10759315
- DOI: 10.1126/scisignal.abc9089
Fluoxetine restrains allergic inflammation by targeting an FcɛRI-ATP positive feedback loop in mast cells
Abstract
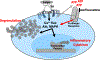
There is a clinical need for new treatment options addressing allergic disease. Selective serotonin reuptake inhibitors (SSRIs) are a class of antidepressants that have anti-inflammatory properties. We tested the effects of the SSRI fluoxetine on IgE-induced function of mast cells, which are critical effectors of allergic inflammation. We showed that fluoxetine treatment of murine or human mast cells reduced IgE-mediated degranulation, cytokine production, and inflammatory lipid secretion, as well as signaling mediated by the mast cell activator ATP. In a mouse model of systemic anaphylaxis, fluoxetine reduced hypothermia and cytokine production. Fluoxetine was also effective in a model of allergic airway inflammation, where it reduced bronchial responsiveness and inflammation. These data show that fluoxetine suppresses mast cell activation by impeding an FcɛRI-ATP positive feedback loop and support the potential repurposing of this SSRI for use in allergic disease.
Conflict of interest statement
Figures







References
-
- Brightling CE et al., Mast-cell infiltration of airway smooth muscle in asthma. N Engl J Med 346, 1699–1705 (2002). - PubMed
-
- Broide DH et al., Evidence of ongoing mast cell and eosinophil degranulation in symptomatic asthma airway. J Allergy Clin Immunol 88, 637–648 (1991). - PubMed
-
- Wang G et al., Sputum mast cell subtypes relate to eosinophilia and corticosteroid response in asthma. Eur Respir J 47, 1123–1133 (2016). - PubMed
-
- Bousquet J et al., Predicting response to omalizumab, an anti-IgE antibody, in patients with allergic asthma. Chest 125, 1378–1386 (2004). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

