A viscoelastic constitutive model for human femoropopliteal arteries
- PMID: 37699504
- PMCID: PMC10802972
- DOI: 10.1016/j.actbio.2023.09.007
A viscoelastic constitutive model for human femoropopliteal arteries
Abstract
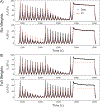
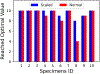
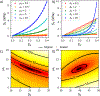
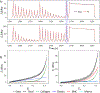
High failure rates present challenges for surgical and interventional therapies for peripheral artery disease of the femoropopliteal artery (FPA). The FPA's demanding biomechanical environment necessitates complex interactions with repair devices and materials. While a comprehensive understanding of the FPA's mechanical characteristics could improve medical treatments, the viscoelastic properties of these muscular arteries remain poorly understood, and the constitutive model describing their time-dependent behavior is absent. We introduce a new viscoelastic constitutive model for the human FPA grounded in its microstructural composition. The model is capable of detailing the contributions of each intramural component to the overall viscoelastic response. Our model was developed utilizing fractional viscoelasticity and tested using biaxial experimental data with hysteresis and relaxation collected from 10 healthy human subjects aged 57 to 65 and further optimized for high throughput and automation. The model accurately described the experimental data, capturing significant nonlinearity and hysteresis that were particularly pronounced circumferentially, and tracked the contribution of passive smooth muscle cells to viscoelasticity that was twice that of the collagen fibers. The high-throughput parameter estimation procedure we developed included a specialized objective function and modifications to enhance convergence for the common exponential-type fiber laws, facilitating computational implementation. Our new model delineates the time-dependent behavior of human FPAs, which will improve the fidelity of computational simulations investigating device-artery interactions and contribute to their greater physical accuracy. Moreover, it serves as a useful tool to investigate the contribution of arterial constituents to overall tissue viscoelasticity, thereby expanding our knowledge of arterial mechanophysiology. STATEMENT OF SIGNIFICANCE: The demanding biomechanical environment of the femoropopliteal artery (FPA) necessitates complex interactions with repair devices and materials, but the viscoelastic properties of these muscular arteries remain poorly understood with the constitutive model describing their time-dependent behavior being absent. We hereby introduce the first viscoelastic constitutive model for the human FPA grounded in its microstructures. This model was tested using biaxial mechanical data collected from 10 healthy human subjects between the ages of 57 to 65. It can detail the contributions of each intramural component to the overall viscoelastic response, showing that the contribution of passive smooth muscle cells to viscoelasticity is twice that of collagen fibers. The usefulness of this model as tool to better understand arterial mechanophysiology was demonstrated.
Keywords: Constitutive modeling; Human femoropopliteal artery; Nonlinear elasticity,; Tissue mechanics; Viscoelasticity.
Copyright © 2023 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures








References
-
- Mahoney EM, Wang K, Keo HH, Duval S, Smolderen KG, Cohen DJ, Steg G, Bhatt DL, Hirsch AT, Vascular hospitalization rates and costs in patients with peripheral artery disease in the United States, Circ. Cardiovasc. Qual. Outcomes 3 (6) (2010) 642–651, doi: 10.1161/CIRCOUTCOMES.109.930735. Publisher: Lippincott Williams & Wilkins Hagerstown, MD; - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

