C. difficile intoxicates neurons and pericytes to drive neurogenic inflammation
- PMID: 37699522
- PMCID: PMC11188852
- DOI: 10.1038/s41586-023-06607-2
C. difficile intoxicates neurons and pericytes to drive neurogenic inflammation
Abstract
Clostridioides difficile infection (CDI) is a major cause of healthcare-associated gastrointestinal infections1,2. The exaggerated colonic inflammation caused by C. difficile toxins such as toxin B (TcdB) damages tissues and promotes C. difficile colonization3-6, but how TcdB causes inflammation is unclear. Here we report that TcdB induces neurogenic inflammation by targeting gut-innervating afferent neurons and pericytes through receptors, including the Frizzled receptors (FZD1, FZD2 and FZD7) in neurons and chondroitin sulfate proteoglycan 4 (CSPG4) in pericytes. TcdB stimulates the secretion of the neuropeptides substance P (SP) and calcitonin gene-related peptide (CGRP) from neurons and pro-inflammatory cytokines from pericytes. Targeted delivery of the TcdB enzymatic domain, through fusion with a detoxified diphtheria toxin, into peptidergic sensory neurons that express exogeneous diphtheria toxin receptor (an approach we term toxogenetics) is sufficient to induce neurogenic inflammation and recapitulates major colonic histopathology associated with CDI. Conversely, mice lacking SP, CGRP or the SP receptor (neurokinin 1 receptor) show reduced pathology in both models of caecal TcdB injection and CDI. Blocking SP or CGRP signalling reduces tissue damage and C. difficile burden in mice infected with a standard C. difficile strain or with hypervirulent strains expressing the TcdB2 variant. Thus, targeting neurogenic inflammation provides a host-oriented therapeutic approach for treating CDI.
© 2023. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing Interests
The authors declare no conflicts of interest.
Figures
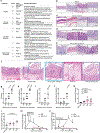





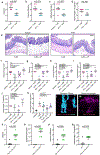

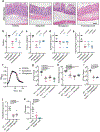





Comment in
-
Clostridioides difficile infection drives neuronal inflammation.Nature. 2023 Oct;622(7983):465-467. doi: 10.1038/d41586-023-02640-3. Nature. 2023. PMID: 37833474 No abstract available.
References
-
- Kelly CP, Pothoulakis C. & LaMont JT Clostridium difficile colitis. N Engl J Med 330, 257–262 (1994). - PubMed
Additional References
-
- Thompson BJ et al. Protective roles of alpha-calcitonin and beta-calcitonin gene-related peptide in spontaneous and experimentally induced colitis. Dig Dis Sci 53, 229–241 (2008). - PubMed
-
- Zhu X, Bergles DE & Nishiyama A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development 135, 145–157 (2008). - PubMed
-
- Cao YQ et al. Primary afferent tachykinins are required to experience moderate to intense pain. Nature 392, 390–394 (1998). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS080833/NS/NINDS NIH HHS/United States
- K08 DK110532/DK/NIDDK NIH HHS/United States
- R01 AI132387/AI/NIAID NIH HHS/United States
- R01 AI139087/AI/NIAID NIH HHS/United States
- R01 NS117626/NS/NINDS NIH HHS/United States
- R01 HL171405/HL/NHLBI NIH HHS/United States
- T32 DK007477/DK/NIDDK NIH HHS/United States
- R01 DK135707/DK/NIDDK NIH HHS/United States
- R01 DK130836/DK/NIDDK NIH HHS/United States
- R01 AI158503/AI/NIAID NIH HHS/United States
- R01 HL150106/HL/NHLBI NIH HHS/United States
- P30 HD018655/HD/NICHD NIH HHS/United States
- P30 DK034854/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

