Development of the TrAnsparent ReportinG of observational studies Emulating a Target trial (TARGET) guideline
- PMID: 37699620
- PMCID: PMC10503363
- DOI: 10.1136/bmjopen-2023-074626
Development of the TrAnsparent ReportinG of observational studies Emulating a Target trial (TARGET) guideline
Abstract
Background: Observational studies are increasingly used to inform health decision-making when randomised trials are not feasible, ethical or timely. The target trial approach provides a framework to help minimise common biases in observational studies that aim to estimate the causal effect of interventions. Incomplete reporting of studies using the target trial framework limits the ability for clinicians, researchers, patients and other decision-makers to appraise, synthesise and interpret findings to inform clinical and public health practice and policy. This paper describes the methods that we will use to develop the TrAnsparent ReportinG of observational studies Emulating a Target trial (TARGET) reporting guideline.
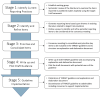
Methods/design: The TARGET reporting guideline will be developed in five stages following recommended guidance. The first stage will identify target trial reporting practices by systematically reviewing published studies that explicitly emulated a target trial. The second stage will identify and refine items to be considered for inclusion in the TARGET guideline by consulting content experts using sequential online surveys. The third stage will prioritise and consolidate key items to be included in the TARGET guideline at an in-person consensus meeting of TARGET investigators. The fourth stage will produce and pilot-test both the TARGET guideline and explanation and elaboration document with relevant stakeholders. The fifth stage will disseminate the TARGET guideline and resources via journals, conferences and courses.
Ethics and dissemination: Ethical approval for the survey has been attained (HC220536). The TARGET guideline will be disseminated widely in partnership with stakeholders to maximise adoption and improve reporting of these studies.
Keywords: EPIDEMIOLOGY; Retrospective Studies; STATISTICS & RESEARCH METHODS.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Cochran WG. Observational studies. Observational Studies 2015;1:126–36. 10.1353/obs.2015.0010 - DOI