Anti-GM-CSF otilimab versus tofacitinib or placebo in patients with active rheumatoid arthritis and an inadequate response to conventional or biologic DMARDs: two phase 3 randomised trials (contRAst 1 and contRAst 2)
- PMID: 37699654
- PMCID: PMC10646845
- DOI: 10.1136/ard-2023-224482
Anti-GM-CSF otilimab versus tofacitinib or placebo in patients with active rheumatoid arthritis and an inadequate response to conventional or biologic DMARDs: two phase 3 randomised trials (contRAst 1 and contRAst 2)
Abstract
Objectives: To investigate the efficacy and safety of otilimab, an antigranulocyte-macrophage colony-stimulating factor antibody, in patients with active rheumatoid arthritis.
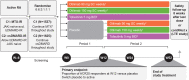
Methods: Two phase 3, double-blind randomised controlled trials including patients with inadequate responses to methotrexate (contRAst 1) or conventional synthetic/biologic disease-modifying antirheumatic drugs (cs/bDMARDs; contRAst 2). Patients received background csDMARDs. Through a testing hierarchy, subcutaneous otilimab (90/150 mg once weekly) was compared with placebo for week 12 endpoints (after which, patients receiving placebo switched to active interventions) or oral tofacitinib (5 mg two times per day) for week 24 endpoints.
Primary endpoint: proportion of patients achieving an American College of Rheumatology response ≥20% (ACR20) at week 12.
Results: The intention-to-treat populations comprised 1537 (contRAst 1) and 1625 (contRAst 2) patients.
Primary endpoint: proportions of ACR20 responders were statistically significantly greater with otilimab 90 mg and 150 mg vs placebo in contRAst 1 (54.7% (p=0.0023) and 50.9% (p=0.0362) vs 41.7%) and contRAst 2 (54.9% (p<0.0001) and 54.5% (p<0.0001) vs 32.5%). Secondary endpoints: in both trials, compared with placebo, otilimab increased the proportion of Clinical Disease Activity Index (CDAI) low disease activity (LDA) responders (not significant for otilimab 150 mg in contRAst 1), and reduced Health Assessment Questionnaire-Disability Index (HAQ-DI) scores. Benefits with tofacitinib were consistently greater than with otilimab across multiple endpoints. Safety outcomes were similar across treatment groups.
Conclusions: Although otilimab demonstrated superiority to placebo in ACR20, CDAI LDA and HAQ-DI, improved symptoms, and had an acceptable safety profile, it was inferior to tofacitinib.
Trial registration numbers: NCT03980483, NCT03970837.
Keywords: autoimmune diseases; biological therapy; cytokines; inflammation; rheumatoid arthritis.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: RMF has received research support from AbbVie, Amgen, Arthrosi, AstraZeneca, Biogen, Bristol Myers Squibb, Boehringer Ingelheim, Galvani, Genentech/Roche, Gilead, GSK, Janssen, Lilly, Novartis, Pfizer, Priovant, Samsung, Sanofi-Genzyme, Selecta and UCB; consulting fees from AbbVie, Amgen, Arthrosi, Bristol Myers Squibb, Boehringer Ingelheim, Celltrion, Galapagos, Galvani, Gilead, GSK, Janssen, Lilly, Novartis, Pfizer, Priovant, Samsung and UCB; has received honoraria from AbbVie, GSK and Pfizer and is an Annals of the Rheumatic Diseases Editorial Board Member. DvdH has received consulting fees from AbbVie, Bayer, Bristol Myers Squibb, Galapagos, Gilead, GSK, Janssen, Lilly, Novartis, Pfizer, UCB Pharma, is Director of Imaging Rheumatology BV and is an Annals of the Rheumatic Diseases Editorial Board Member. VS has received consulting fees from AbbVie, Alpine, Alumis, Amgen, Aria, AstraZeneca, Bayer, Bristol Myers Squibb, Boehringer Ingelheim, Celltrion, Ermium, Genentech/Roche, GSK, Horizon, Inmedix, Janssen, Kiniksa, Lilly, Merck, MiMedx, Novartis, Omeros, Pfizer, R-Pharm, RAPT, Regeneron, Samsung, Sandoz, Sanofi, Scipher, Setpoint, Sorrento, Spherix, Tonix and Urica. TA has accepted research grants and/or honoraria for meetings from AbbVie, Alexion, Astellas Pharma, Bristol-Myers Squibb, Chugai Pharmaceutical, Daiichi Sankyo, Eisai, Gilead Sciences, GSK, Lilly Japan, Mitsubishi Tanabe Pharma, Otsuka Pharmaceutical, Pfizer, Takeda Pharmaceutical, and UCB Japan. IBM has received consultancy and research support from AbbVie, Amgen, AstraZeneca, Bristol Myers Squibb, Causeway, Compugen, Gilead Sciences, GSK, Lilly, Novartis, Pfizer, and UCB and holds a leadership role in Evelo, University of Glasgow, Versus Arthritis, and is an NHS GGC Board Member and an Annals of the Rheumatic Diseases Editorial Board Member. TT received payment or honoraria from AbbVie, Asahi Kasei, Astellas, AstraZeneca K.K., Bristol-Myers Squibb, Chugai Pharmaceutical, Daiichi Sankyo, Eisai, Gilead Sciences, Janssen K.K, Lilly Japan, Mitsubishi-Tanabe, Pfizer Japan and is an Annals of the Rheumatic Diseases Editorial Board Member. PCT has received consulting fees from AbbVie, Biogen, Bristol Myers Squibb, Fresenius, Galapagos, Gilead Sciences, GSK, Janssen, Lilly, Nordic Pharma, Pfizer, Roche, Sanofi, and UCB, and research support from Galapagos. MB, DB, JD, CG, AG, SM, CO’S, DS, LAS, CS, JES, MW, RW and SW are employees of GSK and hold GSK stock/shares. MEW receives research support from AbbVie, Aqtual, Bristol Myers Squibb and Lilly, and consultation fees from AbbVie, Aclaris, Amgen, Bayer, Bristol Myers Squibb, Corvitas, Genosco, Gilead Sciences, GSK, Horizon, Johnson & Johnson, Lilly, Novartis, Pfizer, Rami Therapeutics, R Pharma, Roche, Sanofi, Scipher, Sci Rhom, Set Point and Tremeau. He holds stock/stock options of CanFite, Inmedix, and Scipher.
Figures


References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

