Langerhans cells shape postnatal oral homeostasis in a mechanical-force-dependent but microbiota and IL17-independent manner
- PMID: 37699897
- PMCID: PMC10497507
- DOI: 10.1038/s41467-023-41409-0
Langerhans cells shape postnatal oral homeostasis in a mechanical-force-dependent but microbiota and IL17-independent manner
Abstract
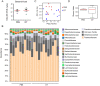
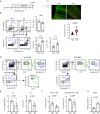
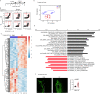
The postnatal interaction between microbiota and the immune system establishes lifelong homeostasis at mucosal epithelial barriers, however, the barrier-specific physiological activities that drive the equilibrium are hardly known. During weaning, the oral epithelium, which is monitored by Langerhans cells (LC), is challenged by the development of a microbial plaque and the initiation of masticatory forces capable of damaging the epithelium. Here we show that microbial colonization following birth facilitates the differentiation of oral LCs, setting the stage for the weaning period, in which adaptive immunity develops. Despite the presence of the challenging microbial plaque, LCs mainly respond to masticatory mechanical forces, inducing adaptive immunity, to maintain epithelial integrity that is also associated with naturally occurring alveolar bone loss. Mechanistically, masticatory forces induce the migration of LCs to the lymph nodes, and in return, LCs support the development of immunity to maintain epithelial integrity in a microbiota-independent manner. Unlike in adult life, this bone loss is IL-17-independent, suggesting that the establishment of oral mucosal homeostasis after birth and its maintenance in adult life involve distinct mechanisms.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Zubeidat K. & Hovav A. H. Shaped by the epithelium - postnatal immune mechanisms of oral homeostasis. Trends Immunol. 42, 622–634 (2021). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

