Peroxisome proliferator-activated receptors as therapeutic target for cancer
- PMID: 37700501
- PMCID: PMC10902584
- DOI: 10.1111/jcmm.17931
Peroxisome proliferator-activated receptors as therapeutic target for cancer
Abstract
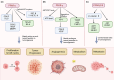
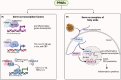
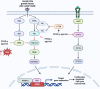
Peroxisome proliferator-activated receptors (PPARs) are transcription factors belonging to the nuclear receptor family. There are three subtypes of PPARs, including PPAR-α, PPAR-β/δ and PPAR-γ. They are expressed in different tissues and act by regulating the expression of target genes in the form of binding to ligands. Various subtypes of PPAR have been shown to have significant roles in a wide range of biological processes including lipid metabolism, body energy homeostasis, cell proliferation and differentiation, bone formation, tissue repair and remodelling. Recent studies have found that PPARs are closely related to tumours. They are involved in cancer cell growth, angiogenesis and tumour immune response, and are essential components in tumour progression and metastasis. As such, they have become a target for cancer therapy research. In this review, we discussed the current state of knowledge on the involvement of PPARs in cancer, including their role in tumourigenesis, the impact of PPARs in tumour microenvironment and the potential of using PPARs combinational therapy to treat cancer by targeting essential signal pathways, or as adjuvants to boost the effects of current chemo and immunotherapies. Our review highlights the complexity of PPARs in cancer and the need for a better understanding of the mechanism in order to design effective cancer therapies.
Keywords: PPARs; cancer; combinational therapy; therapeutic targets; tumor microenvironment.
© 2023 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this paper.
Figures


Similar articles
-
The role of peroxisome proliferator-activated receptors in carcinogenesis and chemoprevention.Nat Rev Cancer. 2012 Feb 9;12(3):181-95. doi: 10.1038/nrc3214. Nat Rev Cancer. 2012. PMID: 22318237 Free PMC article. Review.
-
Biology and therapeutic applications of peroxisome proliferator- activated receptors.Curr Top Med Chem. 2012;12(6):548-84. doi: 10.2174/156802612799436669. Curr Top Med Chem. 2012. PMID: 22242855 Review.
-
Present concepts and future outlook: function of peroxisome proliferator-activated receptors (PPARs) for pathogenesis, progression, and therapy of cancer.J Cell Physiol. 2007 Jul;212(1):1-12. doi: 10.1002/jcp.20998. J Cell Physiol. 2007. PMID: 17443682 Review.
-
Peroxisome proliferator-activated receptors (PPARs) in the control of bone metabolism.Fundam Clin Pharmacol. 2007 Jun;21(3):231-44. doi: 10.1111/j.1472-8206.2007.00486.x. Fundam Clin Pharmacol. 2007. PMID: 17521292 Review.
-
Peroxisome Proliferator-Activated Receptors and the Hallmarks of Cancer.Cells. 2022 Aug 5;11(15):2432. doi: 10.3390/cells11152432. Cells. 2022. PMID: 35954274 Free PMC article. Review.
Cited by
-
The Effect of Eight Weeks of High-Intensity Interval Training on Follistatin Gene Expression in the Fast and Slow Twitch Muscles of Rats with Myocardial Infarction.Iran J Med Sci. 2024 Nov 1;49(11):716-723. doi: 10.30476/ijms.2024.99387.3141. eCollection 2024 Nov. Iran J Med Sci. 2024. PMID: 39678528 Free PMC article.
-
Non-coding RNAs as Key Regulators of the Notch Signaling Pathway in Glioblastoma: Diagnostic, Prognostic, and Therapeutic Targets.CNS Neurol Disord Drug Targets. 2024;23(10):1203-1216. doi: 10.2174/0118715273277458231213063147. CNS Neurol Disord Drug Targets. 2024. PMID: 38279763 Review.
-
Phosphoenolpyruvate carboxykinase 2 (PCK2) attenuates bovine adipocyte lipolysis through PNPLA2 activity repression.Funct Integr Genomics. 2025 Jul 18;25(1):157. doi: 10.1007/s10142-025-01666-2. Funct Integr Genomics. 2025. PMID: 40679709 No abstract available.
-
Breaking Barriers: The Role of the Bone Marrow Microenvironment in Multiple Myeloma Progression.Int J Mol Sci. 2025 Jul 28;26(15):7301. doi: 10.3390/ijms26157301. Int J Mol Sci. 2025. PMID: 40806429 Free PMC article. Review.
-
Role of Peroxisome Proliferator-Activated Receptor α-Dependent Mitochondrial Metabolism in Ovarian Cancer Stem Cells.Int J Mol Sci. 2024 Nov 1;25(21):11760. doi: 10.3390/ijms252111760. Int J Mol Sci. 2024. PMID: 39519311 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

