Two decades of heart regeneration research: Cardiomyocyte proliferation and beyond
- PMID: 37700522
- PMCID: PMC10840678
- DOI: 10.1002/wsbm.1629
Two decades of heart regeneration research: Cardiomyocyte proliferation and beyond
Abstract
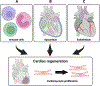
Interest in vertebrate cardiac regeneration has exploded over the past two decades since the discovery that adult zebrafish are capable of complete heart regeneration, contrasting the limited regenerative potential typically observed in adult mammalian hearts. Undercovering the mechanisms that both support and limit cardiac regeneration across the animal kingdom may provide unique insights in how we may unlock this capacity in adult humans. In this review, we discuss key discoveries in the heart regeneration field over the last 20 years. Initially, seminal findings revealed that pre-existing cardiomyocytes are the major source of regenerated cardiac muscle, drawing interest into the intrinsic mechanisms regulating cardiomyocyte proliferation. Moreover, recent studies have identified the importance of intercellular interactions and physiological adaptations, which highlight the vast complexity of the cardiac regenerative process. Finally, we compare strategies that have been tested to increase the regenerative capacity of the adult mammalian heart. This article is categorized under: Cardiovascular Diseases > Stem Cells and Development.
Keywords: cardiomyocyte proliferation; heart regeneration; immune system; stem cells.
© 2023 Wiley Periodicals LLC.
Conflict of interest statement
CONFLICT OF INTERESTS
The authors have no conflicts of interest to disclose.
Figures
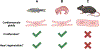


References
-
- Addis RC, Ifkovits JL, Pinto F, Kellam LD, Esteso P, Rentschler S, Christoforou N, Epstein JA, & Gearhart JD (2013). Optimization of Direct Fibroblast Reprogramming to Cardiomyocytes Using Calcium Activity as a Functional Measure of Success. Journal of Molecular and Cellular Cardiology, 60, 97–106. 10.1016/j.yjmcc.2013.04.004 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

