Impact of measurement and feedback on chlorhexidine gluconate bathing among intensive care unit patients: A multicenter study
- PMID: 37700540
- PMCID: PMC10859163
- DOI: 10.1017/ice.2023.177
Impact of measurement and feedback on chlorhexidine gluconate bathing among intensive care unit patients: A multicenter study
Abstract
Objective: To assess whether measurement and feedback of chlorhexidine gluconate (CHG) skin concentrations can improve CHG bathing practice across multiple intensive care units (ICUs).
Design: A before-and-after quality improvement study measuring patient CHG skin concentrations during 6 point-prevalence surveys (3 surveys each during baseline and intervention periods).
Setting: The study was conducted across 7 geographically diverse ICUs with routine CHG bathing.
Participants: Adult patients in the medical ICU.
Methods: CHG skin concentrations were measured at the neck, axilla, and inguinal region using a semiquantitative colorimetric assay. Aggregate unit-level CHG skin concentration measurements from the baseline period and each intervention period survey were reported back to ICU leadership, which then used routine education and quality improvement activities to improve CHG bathing practice. We used multilevel linear models to assess the impact of intervention on CHG skin concentrations.
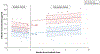
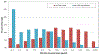
Results: We enrolled 681 (93%) of 736 eligible patients; 92% received a CHG bath prior to survey. At baseline, CHG skin concentrations were lowest on the neck, compared to axillary or inguinal regions (P < .001). CHG was not detected on 33% of necks, 19% of axillae, and 18% of inguinal regions (P < .001 for differences in body sites). During the intervention period, ICUs that used CHG-impregnated cloths had a 3-fold increase in patient CHG skin concentrations as compared to baseline (P < .001).
Conclusions: Routine CHG bathing performance in the ICU varied across multiple hospitals. Measurement and feedback of CHG skin concentrations can be an important tool to improve CHG bathing practice.
Conflict of interest statement
M.K.H. has been a coinvestigator on several research studies for which Sage Products (now part of Stryker Corporation), Molnlycke, and Medline provided chlorhexidine products at no charge to hospitals and skilled nursing facilities participating in the research. Neither M.K.H. nor her employer (Rush University Medical Center) received chlorhexidine products. S.G. is a coinvestigator on a study in which participating hospital and nursing homes received contributed antiseptic product from Stryker (Sage Pharmaceuticals), Clorox, Medline, and Xttrium; companies had no role in the design, conduct, analysis, or publication of these studies. C.R. reports royalties from UpToDate, Inc, and consulting fees from Pfizer and Cytovale for topics unrelated to this study. D.W. was a consultant for Molynlycke Health Care AB after completion of the study. M.Y.L. has received research support in the form of contributed product from Sage Products (now part of Stryker Corporation).
Figures

References
-
- Bleasdale SC, Trick WE, Gonzalez IM, Lyles RD, Hayden MK, Weinstein RA. Effectiveness of chlorhexidine bathing to reduce catheter-associated bloodstream infections in medical intensive care unit patients. Arch Intern Med 2007;167:2073–2079. - PubMed
-
- Supple L, Kumaraswami M, Kundrapu S, et al. Chlorhexidine only works if applied correctly: use of a simple colorimetric assay to provide monitoring and feedback on effectiveness of chlorhexidine application. Infect Control Hosp Epidemiol 2015;36:1095–1097. - PubMed

