Tirzepatide Immunogenicity on Pharmacokinetics, Efficacy, and Safety: Analysis of Data From Phase 3 Studies
- PMID: 37700637
- PMCID: PMC10795913
- DOI: 10.1210/clinem/dgad532
Tirzepatide Immunogenicity on Pharmacokinetics, Efficacy, and Safety: Analysis of Data From Phase 3 Studies
Abstract
Context: Antidrug antibodies (ADA) can potentially affect drug pharmacokinetics, safety, and efficacy.
Objective: This work aimed to evaluate treatment-emergent (TE) ADA in tirzepatide (TZP)-treated participants across 7 phase 3 trials and their potential effect on pharmacokinetics, efficacy, and safety.
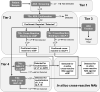
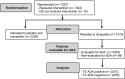
Methods: ADA were assessed at baseline and throughout the study until end point, defined as week 40 (SURPASS-1, -2, and -5) or week 52 (SURPASS-3, -4, Japan-Mono, and Japan-Combo). Samples for ADA characterization were collected at SURPASS trial sites. Participants included ADA-evaluable TZP-treated patients with type 2 diabetes (N = 5025). Interventions included TZP 5, 10, or 15 mg. ADA were detected and characterized for their ability to cross-react with native glucose-dependent insulinotropic polypeptide (nGIP) and glucagon-like peptide-1 (nGLP-1), neutralize tirzepatide activity on GIP and GLP-1 receptors, and neutralize nGIP and nGLP-1.
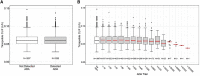
Results: TE ADA developed in 51.1% of tirzepatide-treated patients. Proportions were similar across dose groups. Maximum ADA titers ranged from 1:20 to 1: 81 920 among TE ADA+ patients. Neutralizing antibodies (NAb) against TZP activity on GIP and GLP-1 receptors were observed in 1.9% and 2.1% of patients, respectively. Less than 1.0% of patients had cross-reactive NAb against nGIP or nGLP-1. TE ADA status, ADA titer, and NAb status had no effect on the pharmacokinetics or efficacy of TZP. More TE ADA+ patients experienced hypersensitivity reactions or injection site reactions than TE ADA- patients. The majority of hypersensitivity and injection site reactions were nonserious and nonsevere, and most events occurred and/or resolved irrespective of TE ADA status or titer.
Conclusion: Immunogenicity did not affect TZP pharmacokinetics or efficacy. The majority of hypersensitivity or injection site reactions experienced by TE ADA+ patients were mild to moderate in severity.
Keywords: clinical trial; immunogenicity; incretin therapy; type 2 diabetes.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Endocrine Society.
Figures

References
-
- Rosenstock J, Wysham C, Frías JP, et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet. 2021; 398(10295):143‐155. - PubMed
-
- Frías JP, Davies MJ, Rosenstock J, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021; 385(6):503‐515. - PubMed
-
- Ludvik B, Giorgino F, Jódar E, et al. Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): a randomised, open-label, parallel-group, phase 3 trial. Lancet. 2021; 398(10300):583‐598. - PubMed
-
- Del Prato S, Kahn SE, Pavo I, et al. Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): a randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet. 2021; 398(10313):1811‐1824. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

